Muscle stem cell adaptations to cellular and environmental stress
- PMID: 35151369
- PMCID: PMC8840228
- DOI: 10.1186/s13395-022-00289-6
Muscle stem cell adaptations to cellular and environmental stress
Abstract
Background: Lifelong regeneration of the skeletal muscle is dependent on a rare population of resident skeletal muscle stem cells, also named 'satellite cells' for their anatomical position on the outside of the myofibre and underneath the basal lamina. Muscle stem cells maintain prolonged quiescence, but activate the myogenic programme and the cell cycle in response to injury to expand a population of myogenic progenitors required to regenerate muscle. The skeletal muscle does not regenerate in the absence of muscle stem cells.
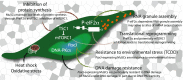
Main body: The notion that lifelong regeneration of the muscle is dependent on a rare, non-redundant population of stem cells seems contradictory to accumulating evidence that muscle stem cells have activated multiple stress response pathways. For example, muscle stem cell quiescence is mediated in part by the eIF2α arm of the integrated stress response and by negative regulators of mTORC1, two translational control pathways that downregulate protein synthesis in response to stress. Muscle stem cells also activate pathways to protect against DNA damage, heat shock, and environmental stress. Here, we review accumulating evidence that muscle stem cells encounter stress during their prolonged quiescence and their activation. While stress response pathways are classically described to be bimodal whereby a threshold dictates cell survival versus cell death responses to stress, we review evidence that muscle stem cells additionally respond to stress by spontaneous activation and fusion to myofibres.
Conclusion: We propose a cellular stress test model whereby the prolonged state of quiescence and the microenvironment serve as selective pressures to maintain muscle stem cell fitness, to safeguard the lifelong regeneration of the muscle. Fit muscle stem cells that maintain robust stress responses are permitted to maintain the muscle stem cell pool. Unfit muscle stem cells are depleted from the pool first by spontaneous activation, or in the case of severe stress, by activating cell death or senescence pathways.
Keywords: MuSC; Muscle stem cell; Stress response pathways; Translational control of gene expression.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Crist CG, Montarras D, Buckingham M. Muscle satellite cells are primed for myogenesis but maintain quiescence with sequestration of Myf5 mRNA targeted by microRNA-31 in mRNP granules. Cell Stem Cell. 2012;11(1):118–126. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

