Immuno-proteomic profiling reveals aberrant immune cell regulation in the airways of individuals with ongoing post-COVID-19 respiratory disease
- PMID: 35151371
- PMCID: PMC8789571
- DOI: 10.1016/j.immuni.2022.01.017
Immuno-proteomic profiling reveals aberrant immune cell regulation in the airways of individuals with ongoing post-COVID-19 respiratory disease
Abstract
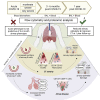
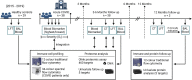
Some patients hospitalized with acute COVID-19 suffer respiratory symptoms that persist for many months. We delineated the immune-proteomic landscape in the airways and peripheral blood of healthy controls and post-COVID-19 patients 3 to 6 months after hospital discharge. Post-COVID-19 patients showed abnormal airway (but not plasma) proteomes, with an elevated concentration of proteins associated with apoptosis, tissue repair, and epithelial injury versus healthy individuals. Increased numbers of cytotoxic lymphocytes were observed in individuals with greater airway dysfunction, while increased B cell numbers and altered monocyte subsets were associated with more widespread lung abnormalities. A one-year follow-up of some post-COVID-19 patients indicated that these abnormalities resolved over time. In summary, COVID-19 causes a prolonged change to the airway immune landscape in those with persistent lung disease, with evidence of cell death and tissue repair linked to the ongoing activation of cytotoxic T cells.
Keywords: COVID-19; SARS-CoV-2; T cells; airways; long COVID; proteomics; respiratory tract; respiratory viral infection; tissue-resident memory.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures






References
-
- Ali M.F., Egan A.M., Shaughnessy G.F., Anderson D.K., Kottom T.J., Dasari H., Van Keulen V.P., Aubry M.C., Yi E.S., Limper A.H., et al. Antifibrotics modify B-cell-induced fibroblast migration and activation in patients with idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2021;64:722–733. - PMC - PubMed
-
- Allden S.J., Ogger P.P., Ghai P., McErlean P., Hewitt R., Toshner R., Walker S.A., Saunders P., Kingston S., Molyneaux P.L., et al. The transferrin receptor CD71 delineates functionally distinct airway macrophage subsets during idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2019;200:209–219. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

