Elicitation of potent SARS-CoV-2 neutralizing antibody responses through immunization with a versatile adenovirus-inspired multimerization platform
- PMID: 35151843
- PMCID: PMC8828441
- DOI: 10.1016/j.ymthe.2022.02.011
Elicitation of potent SARS-CoV-2 neutralizing antibody responses through immunization with a versatile adenovirus-inspired multimerization platform
Abstract
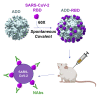
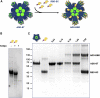
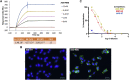
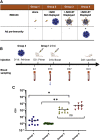
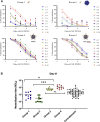
Virus-like particles (VLPs) are highly suited platforms for protein-based vaccines. In the present work, we adapted a previously designed non-infectious adenovirus-inspired 60-mer dodecahedric VLP (ADDomer) to display a multimeric array of large antigens through a SpyTag/SpyCatcher system. To validate the platform as a potential COVID-19 vaccine approach, we decorated the newly designed VLP with the glycosylated receptor binding domain (RBD) of SARS-CoV-2. Cryoelectron microscopy structure revealed that up to 60 copies of this antigenic domain could be bound on a single ADDomer particle, with the symmetrical arrangements of a dodecahedron. Mouse immunization with the RBD decorated VLPs already showed a significant specific humoral response following prime vaccination, greatly reinforced by a single boost. Neutralization assays with SARS-CoV-2 spike pseudo-typed virus demonstrated the elicitation of strong neutralization titers, superior to those of COVID-19 convalescent patients. Notably, the presence of pre-existing immunity against the adenoviral-derived particles did not hamper the immune response against the antigen displayed on its surface. This plug and play vaccine platform represents a promising new highly versatile tool to combat emergent pathogens.
Keywords: Adenovirus; Antigen display; COVID-19; Neutralizing antibodies; Receptor-binding domain; SARS-CoV-2; Vaccine platform; Virus-like particle.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures



References
-
- Fender P., Ruigrok R.W., Gout E., Buffet S., Chroboczek J. Adenovirus dodecahedron, a new vector for human gene transfer. Nat. Biotechnol. 1997;15:52–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

