Interaction of A3 adenosine receptor ligands with the human multidrug transporter ABCG2
- PMID: 35152062
- PMCID: PMC8893036
- DOI: 10.1016/j.ejmech.2022.114103
Interaction of A3 adenosine receptor ligands with the human multidrug transporter ABCG2
Abstract
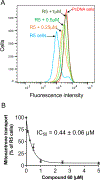
Various adenosine receptor nucleoside-like ligands were found to modulate ATP hydrolysis by the multidrug transporter ABCG2. Both ribose-containing and rigidified (N)-methanocarba nucleosides (C2-, N6- and 5'-modified), as well as adenines (C2-, N6-, and deaza modified), were included. 57 compounds out of 63 tested either stimulated (50) or inhibited (7) basal ATPase activity. Structure-activity analysis showed a separation of adenosine receptor and ABCG2 activities. The 7-deaza modification had favorable effects in both (N)-methanocarba nucleosides and adenines. Adenine 37c (MRS7608) and (N)-methanocarba 7-deaza-5'-ethyl ester 60 (MRS7343) were found to be potent stimulators of ABCG2 ATPase activity with EC50 values of 13.2 ± 1.7 and 13.2 ± 2.2 nM, respectively. Both had affinity in the micromolar range for A3 adenosine receptor and lacked the 5'-amide agonist-enabling group (37c was reported as a weak A3 antagonist, Ki 6.82 μM). Compound 60 significantly inhibited ABCG2 substrate transport (IC50 0.44 μM). Docking simulations predicted the interaction of 60 with 21 residues in the drug-binding pocket of ABCG2.
Keywords: A(3) adenosine receptor; ABC transporter; ABCG2; ATP hydrolysis; Drug transport; Multidrug resistance.
Copyright © 2022. Published by Elsevier Masson SAS.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Structural determinants of A(3) adenosine receptor activation: nucleoside ligands at the agonist/antagonist boundary.J Med Chem. 2002 Sep 26;45(20):4471-84. doi: 10.1021/jm020211+. J Med Chem. 2002. PMID: 12238926 Free PMC article.
-
Evidence for the Interaction of A3 Adenosine Receptor Agonists at the Drug-Binding Site(s) of Human P-glycoprotein (ABCB1).Mol Pharmacol. 2019 Aug;96(2):180-192. doi: 10.1124/mol.118.115295. Epub 2019 May 24. Mol Pharmacol. 2019. PMID: 31127007 Free PMC article.
-
Characterization of drug transport, ATP hydrolysis, and nucleotide trapping by the human ABCG2 multidrug transporter. Modulation of substrate specificity by a point mutation.J Biol Chem. 2002 Dec 13;277(50):47980-90. doi: 10.1074/jbc.M207857200. Epub 2002 Oct 8. J Biol Chem. 2002. PMID: 12374800
-
Ribose modified nucleosides and nucleotides as ligands for purine receptors.Nucleosides Nucleotides Nucleic Acids. 2001 Apr-Jul;20(4-7):333-41. doi: 10.1081/NCN-100002305. Nucleosides Nucleotides Nucleic Acids. 2001. PMID: 11563046 Free PMC article. Review.
-
Multidrug efflux transporter ABCG2: expression and regulation.Cell Mol Life Sci. 2021 Nov;78(21-22):6887-6939. doi: 10.1007/s00018-021-03901-y. Epub 2021 Sep 29. Cell Mol Life Sci. 2021. PMID: 34586444 Free PMC article. Review.
Cited by
-
E. B. Hershberg Award: Taming Inflammation by Tuning Purinergic Signaling.Acc Chem Res. 2025 Mar 18;58(6):958-970. doi: 10.1021/acs.accounts.5c00011. Epub 2025 Mar 5. Acc Chem Res. 2025. PMID: 40043099
-
Network Pharmacology Exploration Reveals Gut Microbiota Modulation as a Common Therapeutic Mechanism for Anti-Fatigue Effect Treated with Maca Compounds Prescription.Nutrients. 2022 Apr 7;14(8):1533. doi: 10.3390/nu14081533. Nutrients. 2022. PMID: 35458095 Free PMC article.
-
Adenosine A3 receptor antagonists as anti-tumor treatment in human prostate cancer: an in vitro study.FEBS Open Bio. 2025 Jul;15(7):1159-1175. doi: 10.1002/2211-5463.70024. Epub 2025 Apr 3. FEBS Open Bio. 2025. PMID: 40181576 Free PMC article.
-
Structure activity relationships of 5-HT2B and 5-HT2C serotonin receptor antagonists: N6, C2 and 5'-Modified (N)-methanocarba-adenosine derivatives.Eur J Med Chem. 2023 Nov 5;259:115691. doi: 10.1016/j.ejmech.2023.115691. Epub 2023 Jul 31. Eur J Med Chem. 2023. PMID: 37562117 Free PMC article.
References
-
- Gottesman MM, Fojo T, Bates SE, Multidrug resistance in cancer: role of ATP-dependent transporters, Nat Rev Cancer, 2 (2002) 48–58. - PubMed
-
- Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM, Biochemical, cellular, and pharmacological aspects of the multidrug transporter, Annu Rev Pharmacol Toxicol, 39 (1999) 361–398. - PubMed
-
- Dean M, Hamon Y, Chimini G, The human ATP-binding cassette (ABC) transporter superfamily, J Lipid Res, 42 (2001) 1007–1017. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

