LRRK2-G2019S mice display alterations in glutamatergic synaptic transmission in midbrain dopamine neurons
- PMID: 35152441
- PMCID: PMC9305867
- DOI: 10.1111/jnc.15588
LRRK2-G2019S mice display alterations in glutamatergic synaptic transmission in midbrain dopamine neurons
Abstract
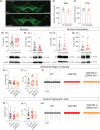
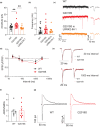
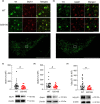
The progressive degeneration of dopamine (DA) neurons in the substantia nigra compacta (SNc) leads to the emergence of motor symptoms in patients with Parkinson's disease (PD). To propose neuroprotective therapies able to slow or halt the progression of the disease, it is necessary to identify cellular alterations that occur before DA neurons degenerate and before the onset of the motor symptoms that characterize PD. Using electrophysiological, histochemical, and biochemical approaches, we have examined if glutamatergic synaptic transmission in DA neurons in the SNc and in the adjacent ventral tegmental area (VTA) was altered in middle-aged (10-12 months old) mice with the hG2019S point mutation (G2019S) in the leucine-rich repeat kinase 2 (LRRK2) gene. G2019S mice showed increased locomotion and exploratory behavior compared with wildtype (WT) littermates, and intact DA neuron integrity. The intrinsic membrane properties and action potential characteristics of DA neurons recorded in brain slices were similar in WT and G2019S mice. Initial glutamate release probability onto SNc-DA neurons, but not VTA-DA neurons, was reduced in G2019S mice. We also found reduced protein amounts of the presynaptic marker of glutamatergic terminals, VGLUT1, and of the GluA1 and GluN1 subunits of AMPA and NMDA receptors, respectively, in the ventral midbrain of G2019S mice. These results identify alterations in glutamatergic synaptic transmission in DA neurons of the SNc and VTA before the onset of motor impairments in the LRRK2-G2019S mouse model of PD.
Keywords: LRRK2-G2019S; Parkinson's disease; dopamine neurons; glutamate; substantia nigra compacta; ventral tegmental area.
© 2022 The Authors. Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Beccano‐Kelly, D. A. , Kuhlmann, N. , Tatarnikov, I. , Volta, M. , Munsie, L. N. , Chou, P. , Cao, L. P. , Han, H. , Tapia, L. , Farrer, M. J. , & Milnerwood, A. J. (2014). Synaptic function is modulated by LRRK2 and glutamate release is increased in cortical neurons of G2019S LRRK2 knock‐in mice. Frontiers in Cellular Neuroscience, 8, 301. - PMC - PubMed
-
- Belluzzi, E. , Gonnelli, A. , Cirnaru, M. D. , Marte, A. , Plotegher, N. , Russo, I. , Civiero, L. , Cogo, S. , Carrion, M. P. , Franchin, C. , Arrigoni, G. , Beltramini, M. , Bubacco, L. , Onofri, F. , Piccoli, G. , & Greggio, E. (2016). LRRK2 phosphorylates pre‐synaptic N‐ethylmaleimide sensitive fusion (NSF) protein enhancing its ATPase activity and SNARE complex disassembling rate. Molecular Neurodegeneration, 11, 1. - PMC - PubMed
-
- Belluzzi, E. , Greggio, E. , & Piccoli, G. (2012). Presynaptic dysfunction in Parkinson's disease: A focus on LRRK2. Biochemical Society Transactions, 40, 1111–1116. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

