RT-PCR negative COVID-19
- PMID: 35152885
- PMCID: PMC8841043
- DOI: 10.1186/s12879-022-07095-x
RT-PCR negative COVID-19
Abstract
Background: COVID-19 is a multi-system infection with emerging evidence-based antiviral and anti-inflammatory therapies to improve disease prognosis. However, a subset of patients with COVID-19 signs and symptoms have repeatedly negative RT-PCR tests, leading to treatment hesitancy. We used comparative serology early in the COVID-19 pandemic when background seroprevalence was low to estimate the likelihood of COVID-19 infection among RT-PCR negative patients with clinical signs and/or symptoms compatible with COVID-19.
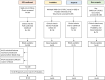
Methods: Between April and October 2020, we conducted serologic testing of patients with (i) signs and symptoms of COVID-19 who were repeatedly negative by RT-PCR ('Probables'; N = 20), (ii) signs and symptoms of COVID-19 but with a potential alternative diagnosis ('Suspects'; N = 15), (iii) no signs and symptoms of COVID-19 ('Non-suspects'; N = 43), (iv) RT-PCR confirmed COVID-19 patients (N = 40), and (v) pre-pandemic samples (N = 55).
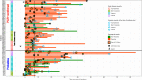
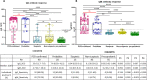
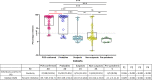
Results: Probables had similar seropositivity and levels of IgG and IgM antibodies as propensity-score matched RT-PCR confirmed COVID-19 patients (60.0% vs 80.0% for IgG, p-value = 0.13; 50.0% vs 72.5% for IgM, p-value = 0.10), but multi-fold higher seropositivity rates than Suspects and matched Non-suspects (60.0% vs 13.3% and 11.6% for IgG; 50.0% vs 0% and 4.7% for IgM respectively; p-values < 0.01). However, Probables were half as likely to receive COVID-19 treatment than the RT-PCR confirmed COVID-19 patients with similar disease severity.
Conclusions: Findings from this study indicate a high likelihood of acute COVID-19 among RT-PCR negative with typical signs/symptoms, but a common omission of COVID-19 therapies among these patients. Clinically diagnosed COVID-19, independent of RT-PCR positivity, thus has a potential vital role in guiding treatment decisions.
Keywords: COVID-19; Diagnosis; RT-PCR; SARS-CoV-2; Suspects.
© 2022. The Author(s).
Conflict of interest statement
D.A. receives royalties and research funding from Cepheid, Inc (Sunnyvale, CA, USA). G.D., M.K., T.R., and M.S. are employed by Beckman Coulter. All other authors have no competing interests.
Figures
References
-
- In Vitro Diagnostics EUAs—Molecular Diagnostic Tests for SARS-CoV-2 https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-em....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

