Novel Compounds Synergize With Venetoclax to Target KMT2A-Rearranged Pediatric Acute Myeloid Leukemia
- PMID: 35153769
- PMCID: PMC8830338
- DOI: 10.3389/fphar.2021.820191
Novel Compounds Synergize With Venetoclax to Target KMT2A-Rearranged Pediatric Acute Myeloid Leukemia
Abstract
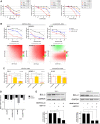
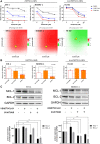
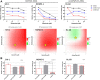
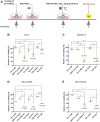
In pediatric acute myeloid leukemia (AML), fusions involving lysine methyltransferase 2A (KMT2A) are considered hallmarks of aggressive AML, for whom the development of targeted specific therapeutic agents to ameliorate classic chemotherapy and obtain a complete eradication of disease is urgent. In this study, we investigated the antiapoptotic proteins in a cohort of 66 pediatric AML patients, finding that 75% of the KMT2A-r are distributed in Q3 + Q4 quartiles of BCL-2 expression, and KMT2A-r have statistically significant high levels of BCL-2, phospho-BCL-2 S70, and MCL-1, indicating a high anti-apoptotic pathway activation. In an attempt to target it, we tested novel drug combinations of venetoclax, a B-cell lymphoma-2 (BCL-2) inhibitor, in KMT2A-MLLT3, for being the most recurrent, and KMT2A-AFDN, for mediating the worst prognosis, rearranged AML cell lines. Our screening revealed that both the bromodomain and extra-terminal domain (BET) inhibitor, I-BET151, and kinase inhibitor, sunitinib, decreased the BCL-2 family protein expression and significantly synergized with venetoclax, enhancing KMT2A-r AML cell line death. Blasts t (6; 11) KMT2A-AFDN rearranged, both from cell lines and primary samples, were shown to be significantly highly responsive to the combination of venetoclax and thioridazine, with the synergy being induced by a dramatic increase of mitochondrial depolarization that triggered blast apoptosis. Finally, the efficacy of novel combined drug treatments was confirmed in KMT2A-r AML cell lines or ex vivo primary KMT2A-r AML samples cultured in a three-dimensional system which mimics the bone marrow niche. Overall, this study identified that, by high-throughput screening, the most KMT2A-selective drugs converged in different but all mitochondrial apoptotic network activation, supporting the use of venetoclax in this AML setting. The novel drug combinations here unveiled provide a rationale for evaluating these combinations in preclinical studies to accelerate the introduction of targeted therapies for the life-threatening KMT2A-AML subgroup of pediatric AML.
Keywords: AML; KMT2A; synergy; targeted drugs; venetoclax.
Copyright © 2022 Tregnago, Benetton, Da Ros, Borella, Longo, Polato, Francescato, Biffi and Pigazzi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Balgobind B. V., Raimondi S. C., Harbott J., Zimmermann M., Alonzo T. A., Auvrignon A., et al. (2009). Novel Prognostic Subgroups in Childhood 11q23/MLL-Rearranged Acute Myeloid Leukemia: Results of an International Retrospective Study. Blood 114, 2489–2496. 10.1182/blood-2009-04-215152 - DOI - PMC - PubMed
-
- Bensi L., Longo R., Vecchi A., Messora C., Garagnani L., Bernardi S., et al. (1995). BCL-2 Oncoprotein Expression in Acute Myeloid Leukemia. Haematologica 80, 98–102. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

