Metformin Ameliorates Inflammation and Airway Remodeling of Experimental Allergic Asthma in Mice by Restoring AMPK α Activity
- PMID: 35153777
- PMCID: PMC8830934
- DOI: 10.3389/fphar.2022.780148
Metformin Ameliorates Inflammation and Airway Remodeling of Experimental Allergic Asthma in Mice by Restoring AMPK α Activity
Erratum in
-
Corrigendum: Metformin Ameliorates Inflammation and Airway Remodeling of Experimental Allergic Asthma in Mice by Restoring AMPKα Activity.Front Pharmacol. 2022 Apr 21;13:900127. doi: 10.3389/fphar.2022.900127. eCollection 2022. Front Pharmacol. 2022. PMID: 35529435 Free PMC article.
Abstract
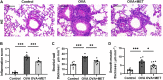
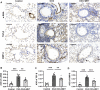
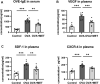
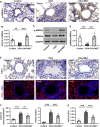
Metformin has been involved in modulating inflammatory state and inhibiting cell proliferation and angiogenesis. This study aimed to determine whether metformin alleviates airway inflammation and remodeling of experimental allergic asthma and elucidate the underlying mechanism. We sensitized and challenged mice with ovalbumin (OVA) to induce allergic asthma. During the challenge period, metformin was administered by intraperitoneal injection. By histopathological and immunohistochemical analyses, metformin-treated mice showed a significant alleviation in airway inflammation, and in the parameters of airway remodeling including goblet cell hyperplasia, collagen deposition and airway smooth muscle hypertrophy compared to those in the OVA-challenged mice. We also observed elevated levels of multiple cytokines (IL-4, IL-5, IL-13, TNF-α, TGF-β1 and MMP-9) in the bronchoalveolar lavage fluid, OVA-specific IgE in the serum and angiogenesis-related factors (VEGF, SDF-1 and CXCR4) in the plasma from asthmatic mice, while metformin reduced all these parameters. Additionally, the activity of 5'-adenosine monophosphate-activated protein kinase a (AMPKα) in the lungs from OVA-challenged mice was remarkably lower than control ones, while after metformin treatment, the ratio of p-AMPKα to AMPKα was upregulated and new blood vessels in the sub-epithelial area as evidenced by CD31 staining were effectively suppressed. These results indicate that metformin ameliorates airway inflammation and remodeling in an OVA-induced chronic asthmatic model and its protective role could be associated with the restoration of AMPKα activity and decreased asthma-related angiogenesis.
Keywords: AMPK; airway inflammation; airway remodeling; asthma; metformin.
Copyright © 2022 Ma, Jin, Guo, Han, Xu, Lu and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- El-Ghaiesh S. H., Bahr H. I., Ibrahiem A. T., Ghorab D., Alomar S. Y., Farag N. E., et al. (2020). Metformin Protects from Rotenone-Induced Nigrostriatal Neuronal Death in Adult Mice by Activating AMPK-FOXO3 Signaling and Mitigation of Angiogenesis. Front. Mol. Neurosci. 13, 84. 10.3389/fnmol.2020.00084 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

