Micro-Technologies for Assessing Microbial Dynamics in Controlled Environments
- PMID: 35154021
- PMCID: PMC8831547
- DOI: 10.3389/fmicb.2021.745835
Micro-Technologies for Assessing Microbial Dynamics in Controlled Environments
Abstract
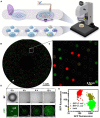
With recent advances in microfabrication technologies, the miniaturization of traditional culturing techniques has provided ideal methods for interrogating microbial communities in a confined and finely controlled environment. Micro-technologies offer high-throughput screening and analysis, reduced experimental time and resources, and have low footprint. More importantly, they provide access to culturing microbes in situ in their natural environments and similarly, offer optical access to real-time dynamics under a microscope. Utilizing micro-technologies for the discovery, isolation and cultivation of "unculturable" species will propel many fields forward; drug discovery, point-of-care diagnostics, and fundamental studies in microbial community behaviors rely on the exploration of novel metabolic pathways. However, micro-technologies are still largely proof-of-concept, and scalability and commercialization of micro-technologies will require increased accessibility to expensive equipment and resources, as well as simpler designs for usability. Here, we discuss three different miniaturized culturing practices; including microarrays, micromachined devices, and microfluidics; advancements to the field, and perceived challenges.
Keywords: microarrays; microfluidics; micromachined devices; nanocultures; unculturable microbes.
Copyright © 2022 Davidson and Niepa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Banks P. (2009). The Microplate Market Past, Present and Future [Online]. Drug Discovery World. Available online at: https://www.ddw-online.com/the-microplate-market-past-present-and-future... (accessed 01 04, 2021)
Publication types
LinkOut - more resources
Full Text Sources

