Aquatic Macrophytes Are Associated With Variation in Biogeochemistry and Bacterial Assemblages of Mountain Lakes
- PMID: 35154025
- PMCID: PMC8828945
- DOI: 10.3389/fmicb.2021.777084
Aquatic Macrophytes Are Associated With Variation in Biogeochemistry and Bacterial Assemblages of Mountain Lakes
Abstract
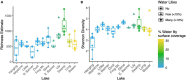
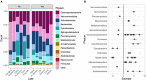
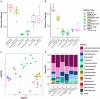
In aquatic systems, microbes likely play critical roles in biogeochemical cycling and ecosystem processes, but much remains to be learned regarding microbial biogeography and ecology. The microbial ecology of mountain lakes is particularly understudied. We hypothesized that microbial distribution among lakes is shaped, in part, by aquatic plant communities and the biogeochemistry of the lake. Specifically, we investigated the associations of yellow water lilies (Nuphar polysepala) with the biogeochemistry and microbial assemblages within mountain lakes at two scales: within a single lake and among lakes within a mountain range. We first compared the biogeochemistry of lakes without water lilies to those colonized to varying degrees by water lilies. Lakes with >10% of the surface occupied by water lilies had lower pH and higher dissolved organic carbon than those without water lilies and had a different microbial composition. Notably, cyanobacteria were negatively associated with water lily presence, a result consistent with the past observation that macrophytes outcompete phytoplankton and can suppress cyanobacterial and algal blooms. To examine the influence of macrophytes on microbial distribution within a lake, we characterized microbial assemblages present on abaxial and adaxial water lily leaf surfaces and in the water column. Microbial diversity and composition varied among all three habitats, with the highest diversity of microbes observed on the adaxial side of leaves. Overall, this study suggests that water lilies influence the biogeochemistry and microbiology of mountains lakes.
Keywords: Nuphar polysepalum; biogeochemistry; macrophytes; microbes; mountain lakes; water lilies.
Copyright © 2022 DeWolf, Calder, Harrison, Randolph, Noren and Weinig.
Conflict of interest statement
BN was employed by the company CellDrop Biosciences Inc., Laramie. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bakker E. S., Van Donk E., Declerck S. A. J., Helmsing N. R., Hidding B., Nolet B. A. (2010). Effect of macrophyte community composition and nutrient enrichment on plant biomass and algal blooms. Basic Appl. Ecol. 11 432–439. 10.1016/j.baae.2010.06.005 - DOI
-
- Bates D., Mächler M., Bolker B., Walker S. (2015). Fitting Linear Mixed-Effects Models using lme4. J. Stat. Softw. 67 1–48,
LinkOut - more resources
Full Text Sources
Miscellaneous

