An Update on Human Papilloma Virus Vaccines: History, Types, Protection, and Efficacy
- PMID: 35154080
- PMCID: PMC8828558
- DOI: 10.3389/fimmu.2021.805695
An Update on Human Papilloma Virus Vaccines: History, Types, Protection, and Efficacy
Abstract
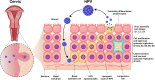
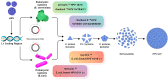
Human papillomavirus (HPV) is the most common sexually transmitted agent worldwide. Early prevention with HPV vaccination is a safe and effective method against this disease. HPV vaccines provided more protection against several oncogenic HPV strains. Three prophylactic HPV vaccines have been approved to target high-risk HPV types and protect against HPV-related disorders. These existing vaccines are based on the recombinant DNA technology and purified L1 protein that is assembled to form HPV empty shells. The prophylactic vaccines are highly immunogenic and can induce production of specific neutralizing antibodies. However, therapeutic vaccines are different from these prophylactic vaccines. They induced cell-mediated immunity against transformed cells, instead of neutralizing antibodies. The second generation of prophylactic HPV vaccines, made from alternative viral components using cost-effective production strategies, is undergoing clinical evaluation. The purpose of this review is to provide a complete and up-to-date review of the types of HPV vaccines and the efficiency of each of them for readers.
Keywords: HPV vaccination; cervical cancer; human papillomavirus (HPV); prevalence; vaccines.
Copyright © 2022 Yousefi, Aria, Ghaedrahmati, Bakhtiari, Azizi, Bastan, Hosseini and Eskandari.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Perez-Campos Mayoral E, Mayoral-Andrade G, Pérez-Campos Mayoral L, Canseco SP, Cruz RM, Hernández-Huerta MT, et al. . Diagnosis of Transient/Latent HPV Infections - A Point of View! Arch Med Res (2018) 49(5):293–6. doi: 10.1016/j.arcmed.2018.10.004 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

