Cytokine Release Syndrome in Cancer Patients Receiving Immune Checkpoint Inhibitors: A Case Series of 25 Patients and Review of the Literature
- PMID: 35154124
- PMCID: PMC8831742
- DOI: 10.3389/fimmu.2022.807050
Cytokine Release Syndrome in Cancer Patients Receiving Immune Checkpoint Inhibitors: A Case Series of 25 Patients and Review of the Literature
Abstract
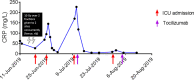
Cytokine release syndrome (CRS) is a phenomenon of immune hyperactivation described in the setting of immunotherapy. Unlike other immune-related adverse events, CRS triggered by immune checkpoint inhibitors (ICIs) is not well described. The clinical characteristics and course of 25 patients with ICI-induced CRS from 2 tertiary hospitals were abstracted retrospectively from the medical records and analyzed. CRS events were confirmed by 2 independent reviewers and graded using the Lee et al. scale. The median duration of CRS was 15.0 days (Q1; Q3 6.3; 29.8) and 10 (40.0%) had multiple episodes of CRS flares. Comparing the clinical factors and biomarkers in Grades 1-2 and 3-5 CRS, we found that patients with Grades 3-5 CRS had following: (i) had longer time to fever onset [25.0 days (Q1; Q3 13.0; 136.5) vs. 3.0 days (Q1; Q3 0.0; 18.0), p=0.027]; (ii) more cardiovascular (p=0.002), neurologic (p=0.001), pulmonary (p=0.044) and rheumatic (p=0.037) involvement; (iii) lower platelet count (p=0.041) and higher urea (p=0.041) at presentation compared to patients with Grades 1-2 CRS. 7 patients (28.0%) with Grades 1-2 CRS were rechallenged using ICIs without event. 9 patients (36.0%) were treated with pulse methylprednisolone and 6 patients (24.0%) were treated with tocilizumab. Despite this, 3 patients (50%) who received tocilizumab had fatal (Grade 5) outcomes from ICI-induced CRS. Longer time to fever onset, lower platelet count and higher urea at presentation were associated with Grade 3-5 CRS. These parameters may be used to predict which patients are likely to develop severe CRS.
Keywords: cytokine release syndrome; immune checkpoint inhibitors; immune-related adverse events; immunotherapy; tocilizumab.
Copyright © 2022 Tay, Toh, Thian, Vellayappan, Fairhurst, Chan, Aminkeng, Bharwani, Huang, Mak and Wong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical