Characterization of Pipefish Immune Cell Populations Through Single-Cell Transcriptomics
- PMID: 35154138
- PMCID: PMC8828949
- DOI: 10.3389/fimmu.2022.820152
Characterization of Pipefish Immune Cell Populations Through Single-Cell Transcriptomics
Abstract
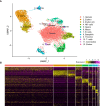
Teleost adaptive immune systems have evolved with more flexibility than previously assumed. A particularly enigmatic system to address immune system modifications in the evolutionary past is represented by the Syngnathids, the family of pipefishes, seahorses and seadragons. These small fishes with their unique male pregnancy have lost the spleen as an important immune organ as well as a functional major histocompatibility class II (MHC II) pathway. How these evolutionary changes have impacted immune cell population dynamics have up to this point remained unexplored. Here, we present the first immune cell repertoire characterization of a syngnathid fish (Syngnathus typhle) using single-cell transcriptomics. Gene expression profiles of individual cells extracted from blood and head-kidney clustered in twelve putative cell populations with eight belonging to those with immune function. Upregulated cell marker genes identified in humans and teleosts were used to define cell clusters. While the suggested loss of CD4+ T-cells accompanied the loss of the MHC II pathway was supported, the upregulation of specific subtype markers within the T-cell cluster indicates subpopulations of regulatory T-cells (il2rb) and cytotoxic T-cells (gzma). Utilizing single-cell RNA sequencing this report is the first to characterize immune cell populations in syngnathids and provides a valuable foundation for future cellular classification and experimental work within the lineage.
Keywords: cell profiling; immune cell; immunity; pipefish; single-cell transcriptomics.
Copyright © 2022 Parker, Guslund, Jentoft and Roth.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

