Long-Term Kinetics of SARS-CoV-2 Antibodies and Impact of Inactivated Vaccine on SARS-CoV-2 Antibodies Based on a COVID-19 Patients Cohort
- PMID: 35154152
- PMCID: PMC8828498
- DOI: 10.3389/fimmu.2022.829665
Long-Term Kinetics of SARS-CoV-2 Antibodies and Impact of Inactivated Vaccine on SARS-CoV-2 Antibodies Based on a COVID-19 Patients Cohort
Abstract
Background: Understanding the long-term kinetic characteristics of SARS-CoV-2 antibodies and the impact of inactivated vaccines on SARS-CoV-2 antibodies in convalescent patients can provide information for developing and improving vaccination strategies in such populations.
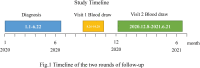
Methods: In this cohort, 402 convalescent patients who tested positive for SARS-CoV-2 by RT-PCR from 1 January to 22 June 2020 in Jiangsu, China, were enrolled. The epidemiological data included demographics, symptom onset, and vaccination history. Blood samples were collected and tested for antibody levels of specific IgG, IgM, RBD-IgG, S-IgG, and neutralizing antibodies using a the commercial magnetic chemiluminescence enzyme immunoassay.
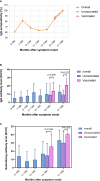
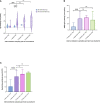
Results: The median follow-up time after symptom onset was 15.6 months (IQR, 14.6 to 15.8). Of the 402 convalescent patients, 44 (13.84%) received an inactivated vaccine against COVID-19. A total of 255 (80.19%) patients were IgG-positive and 65 (20.44%) were IgM-positive. The neutralizing antibody was 83.02%. Compared with non-vaccinated individuals, the IgG antibody levels in vaccinated people were higher (P=0.007). Similarly, antibody levels for RBD-IgG, S-IgG, and neutralizing antibodies were all highly increased in vaccinated individuals (P<0.05). IgG levels were significantly higher after vaccination than before vaccination in the same population. IgG levels in those who received 'single dose and ≥14d' were similar to those with two doses (P>0.05). Similar conclusions were drawn for RBD-IgG and the neutralizing antibody.
Conclusion: 15.6 months after symptom onset, the majority of participants remained positive for serum-specific IgG, RBD-IgG, S-IgG, and neutralizing antibodies. For convalescent patients, a single dose of inactivated vaccine against COVID-19 can further boost antibody titres.
Keywords: SARS-CoV-2; antibody responses; long-term kinetics; natural infection; vaccination.
Copyright © 2022 Zhang, Xu, Li, Zhou, Kong, Peng, Zhu, Bao, Jin, Gao, Zhao and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Who Coronavirus (Covid-19) Dashboard (2021). Available at: https://covid19.who.int/ (Accessed 2021 October 16).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

