Diversity of Dysregulated Long Non-Coding RNAs in HBV-Related Hepatocellular Carcinoma
- PMID: 35154157
- PMCID: PMC8831247
- DOI: 10.3389/fimmu.2022.834650
Diversity of Dysregulated Long Non-Coding RNAs in HBV-Related Hepatocellular Carcinoma
Abstract
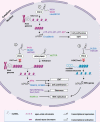
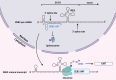
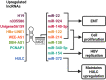
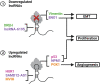
Infection with the hepatitis B virus (HBV) continues to pose a major threat to public health as approximately 292 million people worldwide are currently living with the chronic form of the disease, for which treatment is non-curative. Chronic HBV infections often progress to hepatocellular carcinoma (HCC) which is one of the world's leading causes of cancer-related deaths. Although the process of hepatocarcinogenesis is multifaceted and has yet to be fully elucidated, several studies have implicated numerous long non-coding RNAs (lncRNAs) as contributors to the development of HCC. These host-derived lncRNAs, which are often dysregulated as a consequence of viral infection, have been shown to function as signals, decoys, guides, or scaffolds, to modulate gene expression at epigenetic, transcriptional, post-transcriptional and even post-translational levels. These lncRNAs mainly function to promote HBV replication and oncogene expression or downregulate tumor suppressors. Very few lncRNAs are known to suppress tumorigenesis and these are often downregulated in HCC. In this review, we describe the mechanisms by which lncRNA dysregulation in HBV-related HCC promotes tumorigenesis and cancer progression.
Keywords: HBV-related HCC; ceRNA; epigenetic regulation; lncRNA; miRNA precursor processing; protein interactions; transcriptional regulation.
Copyright © 2022 Samudh, Shrilall, Arbuthnot, Bloom and Ely.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

