Safety evaluation of glucosylated steviol glycosides as a food additive in different food categories
- PMID: 35154440
- PMCID: PMC8826121
- DOI: 10.2903/j.efsa.2022.7066
Safety evaluation of glucosylated steviol glycosides as a food additive in different food categories
Abstract
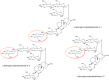
The EFSA Panel on Food Additive and Flavourings (FAF) assessed the safety of glucosylated steviol glycosides proposed for use as a new food additive in different food categories. Glucosylated steviol glycosides consist of a mixture of glucosylated steviol glycosides, containing 1-20 additional glucose units bound to the parent steviol glycosides. Glucosylated steviol glycosides consist of not less than 95% (on dry, dextrin-free, basis) of total steviol glycosides, comprised of glucosylated and parent steviol glycosides. Glucosylated steviol glycosides are produced via enzymatic bioconversion using cyclomaltodextrin glucanotransferase (CGTase) (EC 2.4.1.19), derived from a non-genetically modified strain of Anoxybacillus caldiproteolyticus, that catalyses the transfer of glucose from starch to steviol glycosides mixtures isolated from the dried leaves of Stevia Rebaudiana. The Panel considered that the metabolism of glucosylated steviol glycosides is sufficiently similar to the already authorised steviol glycosides, and thus, the toxicological data previously assessed by the ANS Panel for steviol glycosides (E 960) were considered to support their safety as food additive. The existing acceptable daily intake (ADI) for steviol glycosides (E 960) of 4 mg/kg body weight (bw) per day expressed as steviol can also be applied to glucosylated steviol glycosides. The Panel concluded that there is no safety concern for the use of glucosylated steviol glycosides as a new food additive at the proposed use and use levels. The Panel recommended some modifications to the specifications proposed by the applicant for glucosylated steviol glycosides with respect to the assay, the definition of the proposed new food additive and the proposed maximum limits for arsenic.
Keywords: cyclomaltodextrin glucanotransferase; food additive; glucosylated steviol glycosides; steviol glycosides.
© 2022 Wiley‐VCH Verlag GmbH & Co. KgaA on behalf of the European Food Safety Authority.
Figures
References
-
- EFSA (European Food Safety Authority) , 2005. Opinion of the Scientific Committee on a request from EFSA related to A Harmonised Approach for Risk Assessment of Substances Which are both Genotoxic and Carcinogenic. EFSA Journal 2005;3(10):282, 33 pp. 10.2903/j.efsa.2005.282 - DOI
-
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources) , 2010. Scientific Opinion on safety of steviol glycosides for the proposed uses as a food additive. EFSA Journal 2010;8(4):1537, 85 pp. 10.2903/j.efsa.2010.1537 - DOI
-
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012;10(7):2760, 60 pp. 10.2903/j.efsa.2012.2760 - DOI
-
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources Added to Food) , 2015a. Scientific opinion on the safety of the proposed amendment of the specifications for steviol glycosides (E 960) as a food additive. EFSA Journal 2015;13(12):4316, 29 pp. 10.2903/j.efsa.2015.4316 - DOI
-
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , 2015b. Scientific Opinion on the extension of use of steviol glycosides (E 960) as a food additive. EFSA Journal 2015;13(6):4146, 20 pp. 10.2903/j.efsa.2015.4146 - DOI
LinkOut - more resources
Full Text Sources