Fitness, risk taking, and spatial behavior covary with boldness in experimental vole populations
- PMID: 35154645
- PMCID: PMC8829380
- DOI: 10.1002/ece3.8521
Fitness, risk taking, and spatial behavior covary with boldness in experimental vole populations
Abstract
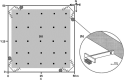
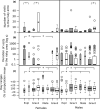
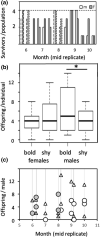
Individuals of a population may vary along a pace-of-life syndrome from highly fecund, short-lived, bold, dispersive "fast" types at one end of the spectrum to less fecund, long-lived, shy, plastic "slow" types at the other end. Risk-taking behavior might mediate the underlying life history trade-off, but empirical evidence supporting this hypothesis is still ambiguous. Using experimentally created populations of common voles (Microtus arvalis)-a species with distinct seasonal life history trajectories-we aimed to test whether individual differences in boldness behavior covary with risk taking, space use, and fitness. We quantified risk taking, space use (via automated tracking), survival, and reproductive success (via genetic parentage analysis) in 8 to 14 experimental, mixed-sex populations of 113 common voles of known boldness type in large grassland enclosures over a significant part of their adult life span and two reproductive events. Populations were assorted to contain extreme boldness types (bold or shy) of both sexes. Bolder individuals took more risks than shyer ones, which did not affect survival. Bolder males but not females produced more offspring than shy conspecifics. Daily home range and core area sizes, based on 95% and 50% Kernel density estimates (20 ± 10 per individual, n = 54 individuals), were highly repeatable over time. Individual space use unfolded differently for sex-boldness type combinations over the course of the experiment. While day ranges decreased for shy females, they increased for bold females and all males. Space use trajectories may, hence, indicate differences in coping styles when confronted with a novel social and physical environment. Thus, interindividual differences in boldness predict risk taking under near-natural conditions and have consequences for fitness in males, which have a higher reproductive potential than females. Given extreme inter- and intra-annual fluctuations in population density in the study species and its short life span, density-dependent fluctuating selection operating differently on the sexes might maintain (co)variation in boldness, risk taking, and pace-of-life.
Keywords: Microtus arvalis; animal personality; automated radio telemetry; behavioral type; fitness; home range; parentage; reproductive success.
© 2022 The Authors. Ecology and Evolution published by John Wiley & Sons Ltd.
Conflict of interest statement
Authors declare that there are no competing interests.
Figures

References
-
- Araya‐Ajoy, Y. G. , Kuhn, S. , Mathot, K. J. , Mouchet, A. , Mutzel, A. , Nicolaus, M. , Wijmenga, J. J. , Kempenaers, B. , & Dingemanse, N. J. (2016). Sources of (co)variation in alternative siring routes available to male great tits (Parus major). Evolution, 70(10), 2308–2321. 10.1111/evo.13024 - DOI - PubMed
-
- Arvidsson, L. K. , Adriaensen, F. , van Dongen, S. , De Stobbeleere, N. , & Matthysen, E. (2017). Exploration behaviour in a different light: Testing cross‐context consistency of a common personality trait. Animal Behaviour, 123, 151–158. 10.1016/j.anbehav.2016.09.005 - DOI
-
- Bates, D. , Mächler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67(1), 1–48. 10.18637/jss.v067.i01 - DOI
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

