Anti-cancer and antimicrobial potential of five soil Streptomycetes: a metabolomics-based study
- PMID: 35154794
- PMCID: PMC8825997
- DOI: 10.1098/rsos.211509
Anti-cancer and antimicrobial potential of five soil Streptomycetes: a metabolomics-based study
Abstract
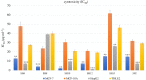
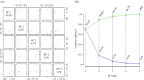
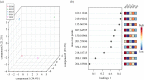
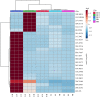
Lack of new anti-cancer and anti-infective agents directed the pharmaceutical research to natural products' discovery especially from actinomycetes as one of the major sources of bioactive compounds. Metabolomics- and dereplication-guided approach has been used successfully in chemical profiling of bioactive actinomycetes. We aimed to study the metabolomic profile of five bioactive actinomycetes to investigate the interesting metabolites responsible for their antimicrobial and anti-cancer activities. Three actinomycetes, namely, Streptomyces sp. SH8, SH10 and SH13, were found to exhibit broad spectrum of antimicrobial activities, whereas isolate SH4 showed the broadest antimicrobial activity against all tested strains. In addition, isolates SH8, SH10 and SH12 displayed potent cytotoxicity against the breast cancer cell line Michigan Cancer Foundation-7 (MCF-7), whereas isolates SH4 and SH12 exhibited potent anti-cancer activity against the hepatoma cell line hepatoma G2 (HepG2) compared with their weak inhibitory properties on the normal breast cells MCF-10A and normal liver cells transformed human liver epithelial-2 (THLE2), respectively. All bioactive isolates were molecularly identified as Streptomyces sp. via 16S rRNA gene sequencing. Our actinobacterial dereplication analysis revealed putative identification of several bioactive metabolites including tetracycline, oxytetracycline and a macrolide antibiotic, novamethymycin. Together, chemical profiling of bioactive Streptomycetes via dereplication and metabolomics helped in assigning their unique metabolites and predicting the bioactive compounds instigating their diverse bioactivities.
Keywords: actinomycetes; anti-cancer; antimicrobial; dereplication; metabolomics; natural products.
© 2022 The Authors.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Sebak M, Saafan AE, Abdelghani S, Bakeer W, Moawad AS, El-Gendy AO. 2021. Isolation and optimized production of putative antimicrobial compounds from Egyptian soil isolate Streptomyces sp. MS. 10. Beni-Suef Univ. J. Basic Appl. Sci. 10, 8. (10.1186/s43088-021-00099-7) - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

