Imaging deeper than the transport mean free path with multiphoton microscopy
- PMID: 35154884
- PMCID: PMC8803047
- DOI: 10.1364/BOE.444696
Imaging deeper than the transport mean free path with multiphoton microscopy
Abstract
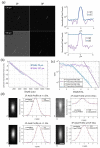
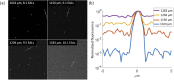
Multiphoton fluorescence microscopy enables deep in vivo imaging by using long excitation wavelengths to increase the penetration depth of ballistic photons and nonlinear excitation to suppress the out-of-focus fluorescence. However, the imaging depth of multiphoton microscopy is limited by tissue scattering and absorption. This fundamental depth limit for two-photon microscopy has been studied theoretically and experimentally. Long wavelength three-photon fluorescence microscopy was developed to image beyond the depth limit of two-photon microscopy and has achieved unprecedented in vivo imaging depth. Here we extend the theoretical framework for characterizing the depth limit of two-photon microscopy to three-photon microscopy. We further verify the theoretical predictions with experimental results from tissue phantoms. We demonstrate experimentally that high spatial resolution diffraction-limited imaging at a depth of 10 scattering mean free paths, which is nearly twice the transport mean free path, is possible with multiphoton microscopy. Our results indicate that the depth limit of three-photon microscopy is significantly beyond what has been achieved in biological tissues so far, and further technological development is required to reach the full potential of three-photon microscopy.
© 2021 Optica Publishing Group under the terms of the Optica Open Access Publishing Agreement.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Bocklitz T., Silge A., Bae H., Rodewald M., Legesse F. B., Meyer T., Popp J., “Non-invasive imaging techniques: from histology to in vivo imaging,” in Molecular Imaging in Oncology , (Springer International Publishing, 2020), pp. 795–812. - PubMed
LinkOut - more resources
Full Text Sources
