Trispecific T-cell engagers for dual tumor-targeting of colorectal cancer
- PMID: 35154908
- PMCID: PMC8837253
- DOI: 10.1080/2162402X.2022.2034355
Trispecific T-cell engagers for dual tumor-targeting of colorectal cancer
Abstract
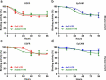
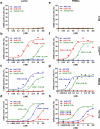
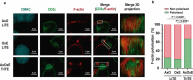
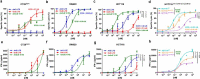
Retargeting of T lymphocytes toward cancer cells by bispecific antibodies has demonstrated its therapeutic potential, with one such antibody approved for the treatment of acute lymphoblastic leukemia (blinatumomab) and several other in clinical trials. However, improvement of their efficacy and selectivity for solid tumors is still required. Here, we describe a novel tandem T-cell recruiting trispecific antibody for the treatment of colorectal cancer (CRC). This construct, termed trispecific T-cell engager (TriTE), consists of a CD3-specific single-chain Fv (scFv) flanked by anti-epidermal growth factor receptor (EGFR) and anti-epithelial cell adhesion molecule (EpCAM) single-domain VHH antibodies. The TriTE was well expressed in mammalian and yeast cells, bound the cognate antigens of the three parental antibodies, and enabled the specific cytolysis of EGFR- and/or EpCAM-expressing cancer cells, without inducing T cell activation and cytoxicity against double-negative (EGFR-EpCAM-) cancer cells. Bivalent bispecific targeting of double-positive HCT116 cells by TriTE improved in vitro potency up to 100-fold compared to single-positive cells and significantly prolonged survival in vivo. In addition, it was less efficient at killing single-positive target cells than the corresponding bispecific controls, leading to potentially enhanced tumor specificity. Moreover, dual targeting of two tumor-associated antigens may contribute toward preventing the tumor escape by antigen loss caused by selective pressures from conventional single-targeting T-cell engagers, and may help to overcome antigenic heterogeneity.
Keywords: Trispecific antibodies; cancer immunotherapy; colorectal cancer; scFv; single-domain antibodies; tandem antibodies.
© 2022 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


References
-
- Compte M, Harwood SL, Muñoz IG, Navarro R, Zonca M, Perez-Chacon G, Erce-Llamazares A, Merino N, Tapia-Galisteo A, Cuesta AM, et al. A Tumor-Targeted Trimeric 4-1BB-Agonistic Antibody Induces Potent Anti-Tumor Immunity without Systemic Toxicity. Nat Commun. 2018;9(1):4809. doi:10.1038/s41467-018-07195-w. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
