Case Report: Durable Clinical Response to Third-Line Pyrotinib After Resistance to Trastuzumab in a Gastric Cancer Patient
- PMID: 35155188
- PMCID: PMC8829539
- DOI: 10.3389/fonc.2021.780577
Case Report: Durable Clinical Response to Third-Line Pyrotinib After Resistance to Trastuzumab in a Gastric Cancer Patient
Abstract
Background: Trastuzumab plus chemotherapy remains the standard first-line treatment strategy for HER2-positive gastric cancer (GC). Trastuzumab resistance, on the other hand, remains a significant issue. There are a few effective anti-HER2 agents for patients who develop resistance to trastuzumab.
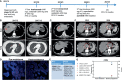
Case presentation: A 49-year-old female was diagnosed with stage IV GC with liver and lung metastasis in July 2017. She underwent gastrostomy, and the immunohistochemistry (IHC) result of postoperative tissue demonstrated HER2 (3+). She received first-line treatment of trastuzumab (440 mg), oxaliplatin (200 mg), and S-1 (40 mg). After treatment for 6 months, the patient achieved complete response (CR) with PFS up to 21 months. After progression, she subsequently received trastuzumab (440 mg) plus oxaliplatin (200 mg) as second-line treatment. However, the patient developed resistance to trastuzumab after 12 months of treatment. She started to receive third-line treatment of irinotecan (200 mg d1) and capecitabine (60 mg bid) plus pyrotinib (400 mg/day). After 2 months of treatment, the tumor is evaluated as partial response with PFS of 12 months.
Conclusions: We presented a patient with HER2-positive GC who benefited from the pyrotinib-based treatment after two lines of trastuzumab-based therapies failed. Further research is required to validate such conclusions.
Keywords: HER2; gastric cancer; pyrotinib; resistance; trastuzumab.
Copyright © 2022 Wu, Li, Qin, Yan, Chen, Jin and Xu.
Conflict of interest statement
Authors ZY and SC were employed by the company 3D Medicines Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Sustained Clinical Benefit of Pyrotinib Combined with Capecitabine Rescue Therapy After Trastuzumab Resistance in HER2-Positive Advanced Gastric Cancer: A Case Report.Onco Targets Ther. 2021 Jul 1;14:3983-3989. doi: 10.2147/OTT.S310421. eCollection 2021. Onco Targets Ther. 2021. PMID: 34234467 Free PMC article.
-
Durable Clinical Response to Pyrotinib After Resistance to Prior Anti-HER2 Therapy for HER2-Positive Advanced Gastric Cancer: A Case Report.Front Oncol. 2019 Dec 20;9:1453. doi: 10.3389/fonc.2019.01453. eCollection 2019. Front Oncol. 2019. PMID: 31956604 Free PMC article.
-
Case Report: Effective Treatment With Pyrotinib and Capecitabine in a Heavily Pretreated Locally Advanced Breast Cancer Harboring Both HER2 Overexpression and Mutant.Front Oncol. 2021 Oct 14;11:715554. doi: 10.3389/fonc.2021.715554. eCollection 2021. Front Oncol. 2021. PMID: 34722261 Free PMC article.
-
Metastatic Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Current Treatment Standards and Future Perspectives.Breast Care (Basel). 2020 Dec;15(6):570-578. doi: 10.1159/000512328. Epub 2020 Nov 12. Breast Care (Basel). 2020. PMID: 33447230 Free PMC article. Review.
-
Efficacy of tucatinib for HER2-positive metastatic breast cancer after HER2-targeted therapy: a network meta-analysis.Future Oncol. 2021 Nov;17(33):4635-4647. doi: 10.2217/fon-2021-0742. Epub 2021 Aug 31. Future Oncol. 2021. PMID: 34463120
Cited by
-
HER2+ advanced gastric cancer: Current state and opportunities (Review).Int J Oncol. 2024 Apr;64(4):36. doi: 10.3892/ijo.2024.5624. Epub 2024 Feb 23. Int J Oncol. 2024. PMID: 38391024 Free PMC article. Review.
-
Targeting ITGβ3 to Overcome Trastuzumab Resistance through Epithelial-Mesenchymal Transition Regulation in HER2-Positive Breast Cancer.Int J Mol Sci. 2024 Aug 8;25(16):8640. doi: 10.3390/ijms25168640. Int J Mol Sci. 2024. PMID: 39201327 Free PMC article.
-
Pyrotinib alone or in combination with docetaxel in refractory HER2-positive gastric cancer: A dose-escalation phase I study.Cancer Med. 2023 May;12(9):10704-10714. doi: 10.1002/cam4.5830. Epub 2023 Apr 20. Cancer Med. 2023. PMID: 37081722 Free PMC article. Clinical Trial.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

