The "Biological Weapons" of Ehrlichia chaffeensis: Novel Molecules and Mechanisms to Subjugate Host Cells
- PMID: 35155275
- PMCID: PMC8834651
- DOI: 10.3389/fcimb.2021.830180
The "Biological Weapons" of Ehrlichia chaffeensis: Novel Molecules and Mechanisms to Subjugate Host Cells
Abstract
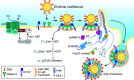
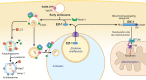
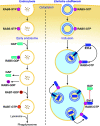
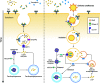
Ehrlichia chaffeensis is an obligatory intracellular bacterium that causes human monocytic ehrlichiosis, an emerging, potentially fatal tick-borne infectious disease. The bacterium enters human cells via the binding of its unique outer-membrane invasin EtpE to the cognate receptor DNase X on the host-cell plasma membrane; this triggers actin polymerization and filopodia formation at the site of E. chaffeensis binding, and blocks activation of phagocyte NADPH oxidase that catalyzes the generation of microbicidal reactive oxygen species. Subsequently, the bacterium replicates by hijacking/dysregulating host-cell functions using Type IV secretion effectors. For example, the Ehrlichia translocated factor (Etf)-1 enters mitochondria and inhibits mitochondria-mediated apoptosis of host cells. Etf-1 also induces autophagy mediated by the small GTPase RAB5, the result being the liberation of catabolites for proliferation inside host cells. Moreover, Etf-2 competes with the RAB5 GTPase-activating protein, for binding to RAB5-GTP on the surface of E. chaffeensis inclusions, which blocks GTP hydrolysis and consequently prevents the fusion of inclusions with host-cell lysosomes. Etf-3 binds ferritin light chain to induce ferritinophagy to obtain intracellular iron. To enable E. chaffeensis to rapidly adapt to the host environment and proliferate, the bacterium must acquire host membrane cholesterol and glycerophospholipids for the purpose of producing large amounts of its own membrane. Future studies on the arsenal of unique Ehrlichia molecules and their interplay with host-cell components will undoubtedly advance our understanding of the molecular mechanisms of obligatory intracellular infection and may identify hitherto unrecognized signaling pathways of human hosts. Such data could be exploited for development of treatment and control measures for ehrlichiosis as well as other ailments that potentially could involve the same host-cell signaling pathways that are appropriated by E. chaffeensis.
Keywords: Ehrlichia chaffeensis; RAB5; ROS; autophagy; ferritinophagy; invasin; membrane cholesterol; type IV secretion effector.
Copyright © 2022 Rikihisa.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Ehrlichia chaffeensis Uses an Invasin To Suppress Reactive Oxygen Species Generation by Macrophages via CD147-Dependent Inhibition of Vav1 To Block Rac1 Activation.mBio. 2020 Apr 21;11(2):e00267-20. doi: 10.1128/mBio.00267-20. mBio. 2020. PMID: 32317318 Free PMC article.
-
Iron robbery by intracellular pathogen via bacterial effector-induced ferritinophagy.Proc Natl Acad Sci U S A. 2021 Jun 8;118(23):e2026598118. doi: 10.1073/pnas.2026598118. Proc Natl Acad Sci U S A. 2021. PMID: 34074773 Free PMC article.
-
Ehrlichia secretes Etf-1 to induce autophagy and capture nutrients for its growth through RAB5 and class III phosphatidylinositol 3-kinase.Autophagy. 2016 Nov;12(11):2145-2166. doi: 10.1080/15548627.2016.1217369. Epub 2016 Aug 19. Autophagy. 2016. PMID: 27541856 Free PMC article.
-
Subversion of RAB5-regulated autophagy by the intracellular pathogen Ehrlichia chaffeensis.Small GTPases. 2019 Sep;10(5):343-349. doi: 10.1080/21541248.2017.1332506. Epub 2017 Jul 5. Small GTPases. 2019. PMID: 28650718 Free PMC article. Review.
-
Molecular Pathogenesis of Ehrlichia chaffeensis Infection.Annu Rev Microbiol. 2015;69:283-304. doi: 10.1146/annurev-micro-091014-104411. Annu Rev Microbiol. 2015. PMID: 26488275 Review.
Cited by
-
Immune evasion strategies of major tick-transmitted bacterial pathogens.Trends Microbiol. 2023 Jan;31(1):62-75. doi: 10.1016/j.tim.2022.08.002. Epub 2022 Aug 31. Trends Microbiol. 2023. PMID: 36055896 Free PMC article. Review.
-
Efficacy and Immune Correlates of OMP-1B and VirB2-4 Vaccines for Protection of Dogs from Tick Transmission of Ehrlichia chaffeensis.mBio. 2022 Dec 20;13(6):e0214022. doi: 10.1128/mbio.02140-22. Epub 2022 Nov 7. mBio. 2022. PMID: 36342170 Free PMC article.
-
Metagenome diversity illuminates the origins of pathogen effectors.mBio. 2024 May 8;15(5):e0075923. doi: 10.1128/mbio.00759-23. Epub 2024 Apr 2. mBio. 2024. PMID: 38564675 Free PMC article.
-
Prevalence of Ehrlichia spp. in dogs and ticks in Hainan Province, China.BMC Vet Res. 2025 Apr 3;21(1):239. doi: 10.1186/s12917-024-04434-9. BMC Vet Res. 2025. PMID: 40176014 Free PMC article.
-
Inhibition of Ehrlichia chaffeensis infection by cell-permeable macrocyclic peptides that bind type IV secretion effector Etf-1.PNAS Nexus. 2023 Jan 27;2(2):pgad017. doi: 10.1093/pnasnexus/pgad017. eCollection 2023 Feb. PNAS Nexus. 2023. PMID: 36874272 Free PMC article.
References
-
- Alrutz M. A., Srivastava A., Wong K. W., D’Souza-Schorey C., Tang M., Ch’Ng L. E., et al. . (2001). Efficient Uptake of Yersinia Pseudotuberculosis via Integrin Receptors Involves a Rac1-Arp 2/3 Pathway That Bypasses N-WASP Function. Mol. Microbiol. 42, 689–703. doi: 10.1046/j.1365-2958.2001.02676.x - DOI - PubMed
-
- Barnewall R. E., Ohashi N., Rikihisa Y. (1999). Ehrlichia chaffeensis and E. sennetsu, But Not the Human Granulocytic Ehrlichiosis Agent, Colocalize With Transferrin Receptor and Up-Regulate Transferrin Receptor mRNA by Activating Iron-Responsive Protein 1. Infect. Immun. 67, 2258–2265. doi: 10.1128/IAI.67.5.2258-2265.1999 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

