Using Proteomic Approaches to Unravel the Response of Ctenocephalides felis felis to Blood Feeding and Infection With Bartonella henselae
- PMID: 35155282
- PMCID: PMC8831700
- DOI: 10.3389/fcimb.2022.828082
Using Proteomic Approaches to Unravel the Response of Ctenocephalides felis felis to Blood Feeding and Infection With Bartonella henselae
Abstract
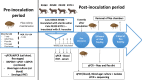
Among the Ctenocephalides felis felis-borne pathogens, Bartonella henselae, the main aetiological agent of cat scratch disease (CSD), is of increasing comparative biomedical importance. Despite the importance of B. henselae as an emergent pathogen, prevention of the diseases caused by this agent in cats, dogs and humans mostly relies on the use of ectoparasiticides. A vaccine targeting both flea fitness and pathogen competence is an attractive choice requiring the identification of flea proteins/metabolites with a dual effect. Even though recent developments in vector and pathogen -omics have advanced the understanding of the genetic factors and molecular pathways involved at the tick-pathogen interface, leading to discovery of candidate protective antigens, only a few studies have focused on the interaction between fleas and flea-borne pathogens. Taking into account the period of time needed for B. henselae replication in flea digestive tract, the present study investigated flea-differentially abundant proteins (FDAP) in unfed fleas, fleas fed on uninfected cats, and fleas fed on B. henselae-infected cats at 24 hours and 9 days after the beginning of blood feeding. Proteomics approaches were designed and implemented to interrogate differentially expressed proteins, so as to gain a better understanding of proteomic changes associated with the initial B. henselae transmission period (24 hour timepoint) and a subsequent time point 9 days after blood ingestion and flea infection. As a result, serine proteases, ribosomal proteins, proteasome subunit α-type, juvenile hormone epoxide hydrolase 1, vitellogenin C, allantoinase, phosphoenolpyruvate carboxykinase, succinic semialdehyde dehydrogenase, glycinamide ribotide transformylase, secreted salivary acid phosphatase had high abundance in response of C. felis blood feeding and/or infection by B. henselae. In contrast, high abundance of serpin-1, arginine kinase, ribosomal proteins, peritrophin-like protein, and FS-H/FSI antigen family member 3 was strongly associated with unfed cat fleas. Findings from this study provide insights into proteomic response of cat fleas to B. henselae infected and uninfected blood meal, as well as C. felis response to invading B. henselae over an infection time course, thus helping understand the complex interactions between cat fleas and B. henselae at protein levels.
Keywords: bartonellosis; cat flea; cat scratch disease; flea-pathogen interface; proteome.
Copyright © 2022 André, Neupane, Lappin, Herrin, Smith, Williams, Collins, Bai, Jorge, Balbuena, Bradley, Maggi and Breitschwerdt.
Conflict of interest statement
RGM is a co-founder and the Chief Technical Officer for Galaxy Diagnostics Inc. In conjunction with Dr. S. Sontakke and North Carolina State University, EBB holds US Patent No. 7,115,385 Media and Methods for Cultivation of Microorganisms, which was issued on October 3rd, 2006. He is a co-founder, shareholder and Chief Scientific Officer for Galaxy Diagnostics, a company that provides advanced diagnostic testing for the detection of Bartonella spp. infections. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

