Crosstalk Between the Gut Microbiota and Epithelial Cells Under Physiological and Infectious Conditions
- PMID: 35155283
- PMCID: PMC8829037
- DOI: 10.3389/fcimb.2022.832672
Crosstalk Between the Gut Microbiota and Epithelial Cells Under Physiological and Infectious Conditions
Abstract
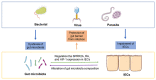
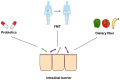
The gastrointestinal tract (GIT) is considered the largest immunological organ, with a diverse gut microbiota, that contributes to combatting pathogens and maintaining human health. Under physiological conditions, the crosstalk between gut microbiota and intestinal epithelial cells (IECs) plays a crucial role in GIT homeostasis. Gut microbiota and derived metabolites can compromise gut barrier integrity by activating some signaling pathways in IECs. Conversely, IECs can separate the gut microbiota from the host immune cells to avoid an excessive immune response and regulate the composition of the gut microbiota by providing an alternative energy source and releasing some molecules, such as hormones and mucus. Infections by various pathogens, such as bacteria, viruses, and parasites, can disturb the diversity of the gut microbiota and influence the structure and metabolism of IECs. However, the interaction between gut microbiota and IECs during infection is still not clear. In this review, we will focus on the existing evidence to elucidate the crosstalk between gut microbiota and IECs during infection and discuss some potential therapeutic methods, including probiotics, fecal microbiota transplantation (FMT), and dietary fiber. Understanding the role of crosstalk during infection may help us to establish novel strategies for prevention and treatment in patients with infectious diseases, such as C. difficile infection, HIV, and COVID-19.
Keywords: COVID-19; epithelial cells; gut microbiota; infection; probiotics.
Copyright © 2022 Zhou, Yuan, Yang, Huang, Li, Li, Yang and Tang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Alvarado D. M., Chen B., Iticovici M., Thaker A. I., Dai N., VanDussen K. L., et al. (2019). Epithelial Indoleamine 2,3-Dioxygenase 1 Modulates Aryl Hydrocarbon Receptor and Notch Signaling to Increase Differentiation of Secretory Cells and Alter Mucus-Associated Microbiota. Gastroenterology 157, 1093–1108.e11. doi: 10.1053/j.gastro.2019.07.013 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

