Tetraphenylethene-Modified Colorimetric and Fluorescent Chemosensor for Hg2+ With Aggregation-Induced Emission Enhancement, Solvatochromic, and Mechanochromic Fluorescence Features
- PMID: 35155382
- PMCID: PMC8828043
- DOI: 10.3389/fchem.2021.811294
Tetraphenylethene-Modified Colorimetric and Fluorescent Chemosensor for Hg2+ With Aggregation-Induced Emission Enhancement, Solvatochromic, and Mechanochromic Fluorescence Features
Abstract
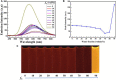
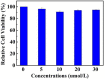
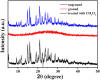
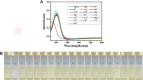
A tetraphenylethene (TPE)-modified rhodanine derivative was successfully designed and prepared, and this luminophor showed intramolecular charge transfer nature from the TPE unit to the rhodanine-3-acetic acid unit. Interestingly, this luminogen not only exhibited typical aggregation-induced emission enhancement (AIEE) behavior but also showed good cell imaging performance. Remarkably, this AIEE-active TPE-containing rhodanine derivative possessed noticeable solvatochromic fluorescence effect involving multiple fluorescent colors of green, yellow-green, yellow, orange, and red. Meanwhile, this fluorescigenic compound displayed reversible mechanochromic fluorescence behavior based on the mutual transformation of between stable crystalline and metastable amorphous states. On the other hand, this multifunctional fluorophor could selectively and sensitively detect Hg2+ in an acetonitrile solution. Furthermore, this chemosensor could also be used to detect Hg2+ on test paper strips.
Keywords: aggregation-induced emission enhancement; chemosensor; mechanochromic fluorescence; rhodanine; solvatochromic fluorescence.
Copyright © 2022 Tian, Deng, Wang, Chen and Pu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








Similar articles
-
Aggregation-induced emission enhancement (AIEE)-active tetraphenylethene (TPE)-based chemosensor for Hg2+ with solvatochromism and cell imaging characteristics.RSC Adv. 2019 Apr 16;9(21):11865-11869. doi: 10.1039/c9ra02119a. eCollection 2019 Apr 12. RSC Adv. 2019. PMID: 35516995 Free PMC article.
-
A 1,3-indandione-functionalized tetraphenylethene: aggregation-induced emission, solvatochromism, mechanochromism, and potential application as a multiresponsive fluorescent probe.Chemistry. 2014 Apr 14;20(16):4661-70. doi: 10.1002/chem.201304174. Epub 2014 Mar 11. Chemistry. 2014. PMID: 24615918
-
Aggregation-induced emission enhancement (AIEE)-active tetraphenylethene (TPE)-based chemosensor for CN.Spectrochim Acta A Mol Biomol Spectrosc. 2021 Jan 15;245:118928. doi: 10.1016/j.saa.2020.118928. Epub 2020 Sep 10. Spectrochim Acta A Mol Biomol Spectrosc. 2021. PMID: 32950857
-
Highly emissive D-A-π-D type aggregation-induced emission (AIE) or aggregation-induced emission enhancement (AIEE)-active benzothiadiazole derivatives with contrasting mechanofluorochromic features.Spectrochim Acta A Mol Biomol Spectrosc. 2022 Jun 5;274:121122. doi: 10.1016/j.saa.2022.121122. Epub 2022 Mar 10. Spectrochim Acta A Mol Biomol Spectrosc. 2022. PMID: 35290941
-
Two AIEE-active α-cyanostilbene derivatives containing BF2 unit for detecting explosive picric acid in aqueous medium.RSC Adv. 2019 Aug 20;9(45):26043-26050. doi: 10.1039/c9ra05116c. eCollection 2019 Aug 19. RSC Adv. 2019. PMID: 35531042 Free PMC article.
References
-
- Bhalla V., Tejpal R., Kumar M. (2010). Rhodamine Appended Terphenyl: A Reversible "Off-On" Fluorescent Chemosensor for Mercury Ions. Sensors Actuators B: Chem. 151 (1), 180–185. 10.1016/j.snb.2010.09.024 - DOI
-
- Chen Z., Liu G., Pu S., Liu S. H. (2017). Carbazole-based Aggregation-Induced Emission (AIE)-active Gold(I) Complex: Persistent Room-Temperature Phosphorescence, Reversible Mechanochromism and Vapochromism Characteristics. Dyes Pigm. 143, 409–415. 10.1016/j.dyepig.2017.05.003 - DOI
-
- Chen Z., Liu G., Pu S., Liu S. H. (2018). Triphenylamine, Carbazole or Tetraphenylethylene-Based Gold(I) Complexes: Tunable Solid-State Room-Temperature Phosphorescence and Various Mechanochromic Luminescence Characteristics. Dyes Pigm. 159, 499–505. 10.1016/j.dyepig.2018.07.016 - DOI
-
- Chen Z., Nie Y., Liu S. H. (2016). Fluorene-based Mononuclear Gold(i) Complexes: the Effect of Alkyl Chain, Aggregation-Induced Emission (AIE) and Mechanochromism Characteristics. RSC Adv. 6 (77), 73933–73938. 10.1039/c6ra17806e - DOI
LinkOut - more resources
Full Text Sources

