Synthesis of N-Heteroarenemethyl Esters via C-C Bond Cleavage of Acyl Cyanides Under Transition Metal-Free Conditions
- PMID: 35155384
- PMCID: PMC8828493
- DOI: 10.3389/fchem.2021.822625
Synthesis of N-Heteroarenemethyl Esters via C-C Bond Cleavage of Acyl Cyanides Under Transition Metal-Free Conditions
Abstract
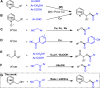
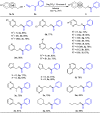
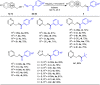
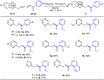
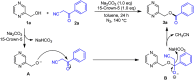
A practical method to synthesize N-heteroaryl esters from N-heteroaryl methanols with acyl cyanides via C-C bond cleavage without using any transition metal is demonstrated here. The use of Na2CO3/15-crown-5 couple enables access to a series of N-heteroaryl esters in high efficiency. This protocol is operationally simple and highly environmentally benign producing only cyanides as byproducts.
Keywords: C-C bond cleavage; N-heteroaryl esters; N-heteroaryl methanols; acyl cyanides; transition-metal free synthesis.
Copyright © 2022 Lai, Su, Hu, Wang, Zhao and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
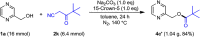

References
-
- Akhlaghinia B., Safaei E., Larki P. (2010). Chemoselective Protocol for O-Acylation with a New Catalyst. Trends Org. Chem. 14, 93–106. 10.1039/toc100814a - DOI
-
- Arzumanyan A. V. (2017). Iron-catalyzed C C Bond Activation/C O Bond Formation: Direct Conversion of Ketones to Esters. Tetrahedron Lett. 58, 4667–4671. 10.1016/j.tetlet.2017.10.068 - DOI
-
- Balalaie S., Mahdidoust M., Eshaghi-Najafabadi R. (2008). ChemInform Abstract: 2-1h-Benzotriazole-1-Yl-1,1,3,3-Tetramethyluronium Tetrafluoro Borate (TBTU) as an Efficient Coupling Reagent for the Esterification of Carboxylic Acids with Alcohols and Phenols at Room Temperature. ChemInform 39, 1141–1144. 10.1002/chin.200839075 - DOI
-
- Bayout I., Bouzemi N., Guo N., Mao X., Serra S., Riva S., et al. (2020). Natural Flavor Ester Synthesis Catalyzed by Lipases. Flavour Fragr J. 35, 209–218. 10.1002/ffj.3554 - DOI
LinkOut - more resources
Full Text Sources

