Fibronectin Adherent Cell Populations Derived From Avascular and Vascular Regions of the Meniscus Have Enhanced Clonogenicity and Differentiation Potential Under Physioxia
- PMID: 35155405
- PMCID: PMC8831898
- DOI: 10.3389/fbioe.2021.789621
Fibronectin Adherent Cell Populations Derived From Avascular and Vascular Regions of the Meniscus Have Enhanced Clonogenicity and Differentiation Potential Under Physioxia
Abstract
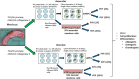
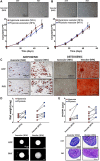
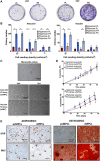
The meniscus is composed of an avascular inner region and vascular outer region. The vascular region has been shown to contain a progenitor population with multilineage differentiation capacity. Strategies facilitating the isolation and propagation of these progenitors can be used to develop cell-based meniscal therapies. Differential adhesion to fibronectin has been used to isolate progenitor populations from cartilage, while low oxygen or physioxia (2% oxygen) enhances the meniscal phenotype. This study aimed to isolate progenitor populations from the avascular and vascular meniscus using differential fibronectin adherence and examine their clonogenicity and differentiation potential under hyperoxia (20% oxygen) and physioxia (2% oxygen). Human vascular and avascular meniscus cells were seeded onto fibronectin-coated dishes for a short period and monitored for colony formation under either hyperoxia or physioxia. Non-fibronectin adherent meniscus cells were also expanded under both oxygen tension. Individual fibronectin adherent colonies were isolated and further expanded, until approximately ten population doublings (passage 3), whereby they underwent chondrogenic, osteogenic, and adipogenic differentiation. Physioxia enhances clonogenicity of vascular and avascular meniscus cells on plastic or fibronectin-coated plates. Combined differential fibronectin adhesion and physioxia isolated a progenitor population from both meniscus regions with trilineage differentiation potential compared to equivalent hyperoxia progenitors. Physioxia isolated progenitors had a significantly enhanced meniscus matrix content without the presence of collagen X. These results demonstrate that combined physioxia and fibronectin adherence can isolate and propagate a meniscus progenitor population that can potentially be used to treat meniscal tears or defects.
Keywords: chondrogenesis; hypoxia; meniscus; meniscus progenitor cells; tissue engineeering.
Copyright © 2022 Pattappa, Reischl, Jahns, Schewior, Lang, Zellner, Johnstone, Docheva and Angele.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Phenotypic characterization of regional human meniscus progenitor cells.Front Bioeng Biotechnol. 2022 Oct 19;10:1003966. doi: 10.3389/fbioe.2022.1003966. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36338137 Free PMC article.
-
The clinical potential of meniscal progenitor cells.J Cartil Jt Preserv. 2024 Dec;4(4):None. doi: 10.1016/j.jcjp.2024.100166. J Cartil Jt Preserv. 2024. PMID: 39669533 Free PMC article. Review.
-
Responses to altered oxygen tension are distinct between human stem cells of high and low chondrogenic capacity.Stem Cell Res Ther. 2016 Oct 20;7(1):154. doi: 10.1186/s13287-016-0419-8. Stem Cell Res Ther. 2016. PMID: 27765063 Free PMC article.
-
Physioxia Promotes the Articular Chondrocyte-Like Phenotype in Human Chondroprogenitor-Derived Self-Organized Tissue.Tissue Eng Part A. 2018 Feb;24(3-4):264-274. doi: 10.1089/ten.TEA.2016.0510. Epub 2017 Jul 7. Tissue Eng Part A. 2018. PMID: 28474537 Free PMC article.
-
Meniscal repair using the outside-to-inside technique.Clin Sports Med. 1996 Jul;15(3):469-81. Clin Sports Med. 1996. PMID: 8800530 Review.
Cited by
-
Insights into chondrocyte populations in cartilaginous tissues at the single-cell level.Nat Rev Rheumatol. 2025 Aug;21(8):465-477. doi: 10.1038/s41584-025-01275-0. Epub 2025 Jul 10. Nat Rev Rheumatol. 2025. PMID: 40640375 Review.
-
Fibronectin and vitronectin alleviate adipose-derived stem cells senescence during long-term culture through the AKT/MDM2/P53 pathway.Sci Rep. 2024 Jun 20;14(1):14242. doi: 10.1038/s41598-024-65339-z. Sci Rep. 2024. PMID: 38902430 Free PMC article.
-
Phenotypic characterization of regional human meniscus progenitor cells.Front Bioeng Biotechnol. 2022 Oct 19;10:1003966. doi: 10.3389/fbioe.2022.1003966. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36338137 Free PMC article.
-
Enhancing osteogenic differentiation of diabetic tendon stem/progenitor cells through hyperoxia: Unveiling ROS/HIF-1α signalling axis.J Cell Mol Med. 2024 Oct;28(20):e70127. doi: 10.1111/jcmm.70127. J Cell Mol Med. 2024. PMID: 39467998 Free PMC article.
-
The clinical potential of meniscal progenitor cells.J Cartil Jt Preserv. 2024 Dec;4(4):None. doi: 10.1016/j.jcjp.2024.100166. J Cartil Jt Preserv. 2024. PMID: 39669533 Free PMC article. Review.
References
-
- Adesida A. B., Grady L. M., Khan W. S., Millward-Sadler S. J., Salter D. M., Hardingham T. E. (2007). Human Meniscus Cells Express Hypoxia Inducible Factor-1α and Increased SOX9 in Response to Low Oxygen Tension in Cell Aggregate Culture. Arthritis Res. Ther. 9 (4), R69. 10.1186/ar2267 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

