Downregulation of NAGLU in VEC Increases Abnormal Accumulation of Lysosomes and Represents a Predictive Biomarker in Early Atherosclerosis
- PMID: 35155448
- PMCID: PMC8826576
- DOI: 10.3389/fcell.2021.797047
Downregulation of NAGLU in VEC Increases Abnormal Accumulation of Lysosomes and Represents a Predictive Biomarker in Early Atherosclerosis
Abstract
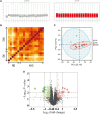
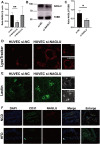
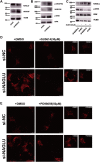
Cardiovascular diseases (CVDs), predominantly caused by atherosclerosis (AS), are the leading cause of mortality worldwide. Although a great number of previous studies have attempted to reveal the molecular mechanism of AS, the underlying mechanism has not been fully elucidated. The aberrant expression profiling of vascular endothelial cells (VECs) gene in early atherosclerosis (EAS) was analyzed according to the dataset (GSE132651) downloaded from the Gene Expression Omnibus (GEO) database. We primarily performed functional annotation analysis on the downregulated genes (DRGs). We further identified that α-N-acetylglucosaminidase (NAGLU), one of the DRGs, played a critical role in the progression of EAS. NAGLU is a key enzyme for the degradation of heparan sulfate (HS), and its deficiency could cause lysosomal accumulation and lead to dysfunctions of VECs. We found that siRNA knockdown of NAGLU in human umbilical vein endothelial cell (HUVEC) aggravated the abnormal accumulation of lysosomes and HS. In addition, the expression of NAGLU was reduced in the EAS model constructed by ApoE -/- mice. Furthermore, we also showed that heparin-binding EGF-like growth factor (HB-EGF) protein was upregulated while NAGLU knockdown in HUVEC could specifically bind to vascular endothelial growth factor receptor 2 (VEGFR2) and promote its phosphorylation, ultimately activating the phosphorylation levels of extracellular signal-regulated kinases (ERKs). However, the application of selective VEGFR2 and ERKs inhibitors, SU5614 and PD98059, respectively, could reverse the abnormal lysosomal storage caused by NAGLU knockdown. These results indicated that downregulation of NAGLU in HUVEC increases the abnormal accumulation of lysosomes and may be a potential biomarker for the diagnosis of EAS.
Keywords: ERK; NAGLU; VEGFR2; bioinformatics analysis; early atherosclerosis; lysosome; vascular endothelial cell.
Copyright © 2022 Xing, Jiang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
EGFR activation triggers cellular hypertrophy and lysosomal disease in NAGLU-depleted cardiomyoblasts, mimicking the hallmarks of mucopolysaccharidosis IIIB.Cell Death Dis. 2018 Jan 18;9(2):40. doi: 10.1038/s41419-017-0187-0. Cell Death Dis. 2018. PMID: 29348482 Free PMC article.
-
Differential Uptake of NAGLU-IGF2 and Unmodified NAGLU in Cellular Models of Sanfilippo Syndrome Type B.Mol Ther Methods Clin Dev. 2019 May 24;14:56-63. doi: 10.1016/j.omtm.2019.05.008. eCollection 2019 Sep 13. Mol Ther Methods Clin Dev. 2019. PMID: 31309128 Free PMC article.
-
The Murine Model of Mucopolysaccharidosis IIIB Develops Cardiopathies over Time Leading to Heart Failure.PLoS One. 2015 Jul 6;10(7):e0131662. doi: 10.1371/journal.pone.0131662. eCollection 2015. PLoS One. 2015. PMID: 26147524 Free PMC article.
-
Molecular genetics of mucopolysaccharidosis type IIIA and IIIB: Diagnostic, clinical, and biological implications.Hum Mutat. 2001 Oct;18(4):264-81. doi: 10.1002/humu.1189. Hum Mutat. 2001. PMID: 11668611 Review.
-
Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress.J Adv Res. 2021 Jul 6;34:43-63. doi: 10.1016/j.jare.2021.06.023. eCollection 2021 Dec. J Adv Res. 2021. PMID: 35024180 Free PMC article. Review.
Cited by
-
Construction and Evaluation of a Risk Score Model for Lymph Node Metastasis-Associated Circadian Clock Genes in Esophageal Squamous Carcinoma.Cells. 2022 Oct 31;11(21):3432. doi: 10.3390/cells11213432. Cells. 2022. PMID: 36359828 Free PMC article.
-
Explore the role of long noncoding RNAs and mRNAs in intracranial atherosclerotic stenosis: From the perspective of neutrophils.Brain Circ. 2023 Nov 30;9(4):240-250. doi: 10.4103/bc.bc_63_23. eCollection 2023 Oct-Dec. Brain Circ. 2023. PMID: 38284107 Free PMC article.
-
Comparative proteomic analysis of plasma exosomes reveals the functional contribution of N-acetyl-alpha-glucosaminidase to Parkinson's disease.Neural Regen Res. 2025 Oct 1;20(10):2998-3012. doi: 10.4103/NRR.NRR-D-23-01500. Epub 2024 Jul 10. Neural Regen Res. 2025. PMID: 38993127 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

