Turning the Curve Into Straight: Phenogenetics of the Spine Morphology and Coordinate Maintenance in the Zebrafish
- PMID: 35155449
- PMCID: PMC8826430
- DOI: 10.3389/fcell.2021.801652
Turning the Curve Into Straight: Phenogenetics of the Spine Morphology and Coordinate Maintenance in the Zebrafish
Abstract
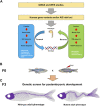
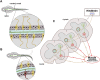
The vertebral column, or spine, provides mechanical support and determines body axis posture and motion. The most common malformation altering spine morphology and function is adolescent idiopathic scoliosis (AIS), a three-dimensional spinal deformity that affects approximately 4% of the population worldwide. Due to AIS genetic heterogenicity and the lack of suitable animal models for its study, the etiology of this condition remains unclear, thus limiting treatment options. We here review current advances in zebrafish phenogenetics concerning AIS-like models and highlight the recently discovered biological processes leading to spine malformations. First, we focus on gene functions and phenotypes controlling critical aspects of postembryonic aspects that prime in spine architecture development and straightening. Second, we summarize how primary cilia assembly and biomechanical stimulus transduction, cerebrospinal fluid components and flow driven by motile cilia have been implicated in the pathogenesis of AIS-like phenotypes. Third, we highlight the inflammatory responses associated with scoliosis. We finally discuss recent innovations and methodologies for morphometrically characterize and analyze the zebrafish spine. Ongoing phenotyping projects are expected to identify novel and unprecedented postembryonic gene functions controlling spine morphology and mutant models of AIS. Importantly, imaging and gene editing technologies are allowing deep phenotyping studies in the zebrafish, opening new experimental paradigms in the morphometric and three-dimensional assessment of spinal malformations. In the future, fully elucidating the phenogenetic underpinnings of AIS etiology in zebrafish and humans will undoubtedly lead to innovative pharmacological treatments against spinal deformities.
Keywords: CSF-cNs; Reissner fiber; cerebrospinal fluid; cilia; inflammation; scoliosis; spine; zebrafish.
Copyright © 2022 Muñoz-Montecinos, Romero, Sepúlveda, Vira, Fehrmann-Cartes, Marcellini, Aguilera, Caprile and Fuentes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Acaroglu R. E., Akel A., Alanay M., Yazici M., Marcucio R. (2009). Comparison of the melatonin and calmodulin in paravertebral muscle and platelets of patients with or without adolescent ıdiopathic scoliosis. Spine J. 18, E659-63. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

