The Role of Disease Severity and Demographics in the Clinical Course of COVID-19 Patients Treated With Convalescent Plasma
- PMID: 35155458
- PMCID: PMC8826061
- DOI: 10.3389/fmed.2021.707895
The Role of Disease Severity and Demographics in the Clinical Course of COVID-19 Patients Treated With Convalescent Plasma
Abstract
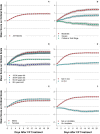
Treatment of patients with COVID-19 using convalescent plasma from recently recovered patients has been shown to be safe, but the time course of change in clinical status following plasma transfusion in relation to baseline disease severity has not yet been described. We analyzed short, descriptive daily reports of patient status in 7,180 hospitalized recipients of COVID-19 convalescent plasma in the Mayo Clinic Expanded Access Program. We assessed, from the day following transfusion, whether the patient was categorized by his or her physician as better, worse or unchanged compared to the day before, and whether, on the reporting day, the patient received mechanical ventilation, was in the ICU, had died or had been discharged. Most patients improved following transfusion, but clinical improvement was most notable in mild to moderately ill patients. Patients classified as severely ill upon enrollment improved, but not as rapidly, while patients classified as critically ill/end-stage and patients on ventilators showed worsening of disease status even after treatment with convalescent plasma. Patients age 80 and over showed little or no clinical improvement following transfusion. Clinical status at the time of convalescent plasma treatment and age appear to be the primary factors in determining the therapeutic effectiveness of COVID-19 convalescent plasma among hospitalized patients.
Keywords: COVID-19; SARS-CoV-2; antibodies; antibody therapy; convalescent plasma therapy.
Copyright © 2022 Ma, Wiggins, Kornatowski, Hailat, Clayburn, Guo, Johnson, Senefeld, Klassen, Baker, Bruno, Fairweather, Wright, Carter, Li, Joyner and Paneth.
Conflict of interest statement
This study received funding from Millennium Pharmaceuticals, Octapharma USA, Inc. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer VB declared a shared affiliation, with the authors CW, AC, WG, JS, SK, SB, RW, and MJ to the handling editor at the time of the review.
Figures
Update of
-
The Role of Disease Severity and Demographics in the Clinical Course of COVID-19 Patients Treated with Convalescent Plasma.medRxiv [Preprint]. 2021 Jan 20:2021.01.19.21249678. doi: 10.1101/2021.01.19.21249678. medRxiv. 2021. Update in: Front Med (Lausanne). 2022 Jan 26;8:707895. doi: 10.3389/fmed.2021.707895. PMID: 33501470 Free PMC article. Updated. Preprint.
References
-
- U.S. Department of Health & Human Services . CDC COVID Data Tracker. (2020). Available online at: https://covid.cdc.gov/covid-data-tracker/#trends_dailycases (accessed March 31, 2021).
-
- Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw F-M, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. (2014) 211:80–90. 10.1093/infdis/jiu396 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

