Investigation of Correlations Between Optical Coherence Tomography Biomarkers and Visual Acuity in X-Linked Retinoschisis
- PMID: 35155459
- PMCID: PMC8828641
- DOI: 10.3389/fmed.2021.734888
Investigation of Correlations Between Optical Coherence Tomography Biomarkers and Visual Acuity in X-Linked Retinoschisis
Abstract
Purpose: To investigate the imaging biomarkers of spectral-domain optical coherence tomography (SD-OCT) and their correlations with age and best-corrected visual acuity (BCVA) in patients with X-linked retinoschisis (XLRS).
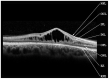
Methods: OCT images of 72 eyes of 39 patients with confirmed XLRS were obtained to assess imaging biomarkers, including but not limited to the automatic evaluation of foveal thickness, central subfield thickness (CST), macular volume, and the manual measurement of area of macular schisis cavity (AMS). Correlations between age/BCVA and all OCT parameters were computed as well.
Results: In this study, median age was 10.5 (8-12) years old and median BCVA was 0.90 (0.70-1.00) logarithm of the minimum angle of resolution. Macular retinoschisis was found in all affected eyes, with peripheral retinoschisis (PRS) in 34 (47.2%) eyes. Cystic cavities most frequently affected inner nuclear layer (100%) in the macula. Ellipsoid zone (EZ) defects occurred in 53 (73.6%) eyes. As for correlation, BCVA was significantly correlated with several OCT parameters, including CST, AMS, EZ defect, PRS and vitreomacular adhesion, whereas no correlation was found between age and any OCT parameter.
Conclusion: Explicable OCT imaging biomarkers such as CST, AMS, and photoreceptor defects were identified and may serve as reference parameters or potential regions of interest for future observational and interventional research design and result interpretation.
Keywords: X-linked retinoschisis; biomarker; correlation; optical coherence tomography; visual acuity.
Copyright © 2022 Lin, Zang, Maman Lawali, Xiao, Zeng, Yu and Hu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Phenotypic Characteristics of a French Cohort of Patients with X-Linked Retinoschisis.Ophthalmology. 2018 Oct;125(10):1587-1596. doi: 10.1016/j.ophtha.2018.03.057. Epub 2018 May 5. Ophthalmology. 2018. PMID: 29739629
-
Wide-field spectral-domain optical coherence tomography in patients and carriers of X-linked retinoschisis.Ophthalmology. 2013 Jan;120(1):169-74. doi: 10.1016/j.ophtha.2012.07.051. Epub 2012 Sep 23. Ophthalmology. 2013. PMID: 23009889 Free PMC article.
-
X-Linked Retinoschisis: Deep Phenotyping and Genetic Characterization.Ophthalmology. 2022 May;129(5):542-551. doi: 10.1016/j.ophtha.2021.11.019. Epub 2021 Nov 23. Ophthalmology. 2022. PMID: 34822951
-
Visual Acuity in Retinal Vein Occlusion, Diabetic, and Uveitic Macular Edema: Central Subfield Thickness and Ellipsoid Zone Analysis.Ophthalmol Retina. 2021 Jul;5(7):633-647. doi: 10.1016/j.oret.2020.10.016. Epub 2020 Oct 29. Ophthalmol Retina. 2021. PMID: 33130256 Clinical Trial.
-
Outcome measures in juvenile X-linked retinoschisis: A systematic review.Eye (Lond). 2020 Oct;34(10):1760-1769. doi: 10.1038/s41433-020-0848-6. Epub 2020 Apr 20. Eye (Lond). 2020. PMID: 32313171 Free PMC article.
Cited by
-
Genotype-Phenotype Associations in an X-Linked Retinoschisis Patient Cohort: The Molecular Dynamic Insight and a Promising SD-OCT Indicator.Invest Ophthalmol Vis Sci. 2024 Feb 1;65(2):17. doi: 10.1167/iovs.65.2.17. Invest Ophthalmol Vis Sci. 2024. PMID: 38324300 Free PMC article.
-
Deep Learning with Automatic Data Augmentation for Segmenting Schisis Cavities in the Optical Coherence Tomography Images of X-Linked Juvenile Retinoschisis Patients.Diagnostics (Basel). 2023 Sep 24;13(19):3035. doi: 10.3390/diagnostics13193035. Diagnostics (Basel). 2023. PMID: 37835778 Free PMC article.
-
Morphological and functional parameters in X-linked retinoschisis patients-A multicentre retrospective cohort study.Front Med (Lausanne). 2024 Jan 11;10:1331889. doi: 10.3389/fmed.2023.1331889. eCollection 2023. Front Med (Lausanne). 2024. PMID: 38351967 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

