Systematic Review and Meta-Analysis of COVID-19 Vaccination Acceptance
- PMID: 35155467
- PMCID: PMC8828741
- DOI: 10.3389/fmed.2021.783982
Systematic Review and Meta-Analysis of COVID-19 Vaccination Acceptance
Abstract
Introduction: Vaccination is an essential intervention to curb the coronavirus disease 2019 (COVID-19) pandemic. This review aimed to estimate the pooled proportion of COVID-19 vaccine acceptance worldwide.
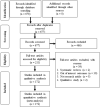
Methods: A systematic search of the MEDLINE (PubMed) database using "COVID-19," "vaccine" and "acceptance" to obtain original research articles published between 2020 and July 2021. Only studies with full text and that were published in English were included. The Joanna Briggs Institute meta-analysis was used to assess the data quality. The meta-analysis was performed using generic inverse variance with a random-effects model using the Review Manager software.
Results: A total of 172 studies across 50 countries worldwide were included. Subgroup analyses were performed with regard to vaccine acceptance, regions, population, gender, vaccine effectiveness, and survey time. The pooled proportion of COVID-19 vaccine acceptance was 61% (95% CI: 59, 64). It was higher in Southeast Asia, among healthcare workers, in males, for vaccines with 95% effectiveness, and during the first survey.
Conclusion: COVID-19 vaccine acceptance needs to be increased to achieve herd immunity to protect the population from the disease. It is crucial to enhance public awareness of COVID-19 vaccination and improve access to vaccines.
Systematic review registration: PROSPERO 2021, identifier CRD42021268645.
Keywords: COVID-19; acceptance; meta-analysis; prevalence; vaccine.
Copyright © 2022 Norhayati, Che Yusof and Azman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
-
- WHO . Weekly Epidemiological Update On COVID-19-31 August 2021. Edition 55 ed. World Health Organization; (2021).
-
- WHO . Tracking SARS-CoV-2 Variants. World Health Organization; (2021).
-
- WHO . Status of COVID-19 Vaccines Within WHO EUL/PQ Evaluation Process. World Health Organization; (2021).
-
- Mayo . Herd Immunity and COVID-19 (coronavirus): What You Need To Know. Mayo Foundation for Medical Education and Research (MFMER). (2021). Available online at: https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/herd... (accessed July 14, 2021).
Publication types
LinkOut - more resources
Full Text Sources