Influence of Nanofillers on Adhesion Properties of Polymeric Composites
- PMID: 35155882
- PMCID: PMC8829956
- DOI: 10.1021/acsomega.1c05448
Influence of Nanofillers on Adhesion Properties of Polymeric Composites
Abstract
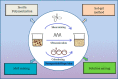
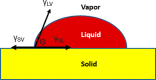
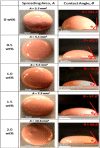
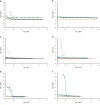
Nanofillers (NFs) are becoming a ubiquitous choice for applications in different technological innovations in various fields, from biomedical devices to automotive product portfolios. Potential physical attributes like large surface areas, high surface energy, and lower structural imperfections make NFs a popular filler over microfillers. One specific application, where NFs are finding applications, is in adhesive science and technology. Incorporating NFs in the adhesive matrix is seen to tune the adhesives' different properties like wettability, rheology, etc. Additionally, the functional benefits (like electrical/thermal conductivity) of these NFs are translated into the adhesives' properties. Such an improvement in the properties is far to achieve using microfillers in the adhesive matrix. This mini-review provides an account of the impact of the addition of various nanofillers (NFs) on the properties of the adhesive composition.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
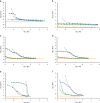
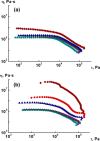
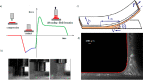


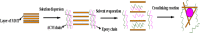
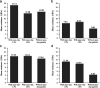
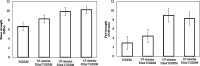
References
-
- Saha S.; Mondal T.; Bhowmick A. K. Pressure Sensitive Adhesive for Healthcare Applications. In Reference Module in Materials Science and Materials Engineering; Elsevier, 2021;10.1016/B978-0-12-820352-1.00106-1. - DOI
- Raihan R.; Elenchezhian M. R. P.; Vadlamudi V. Chapter 11- Durability of bonded composite systems. In Durability of Composite Systems, 1st ed.; Woodhead Publishing Series in Composites Science and Engineering; Reifsnider K. L., Ed.; Elsevier, 2020; pp 383–401.
- Ebnesajjad S.; Landrock A. H. Chapter 4: Classification of adhesives and compounds. In Adhesives Technology Handbook, 3rd ed.; Ebnesajjad S., Landrock A. H., Eds.; Elsevier, 2014; pp 67–83.
- Schultz J.; Nardin M. Chapter 3: Theories and Mechanisms of Adhesion. In Handbook of Adhesive Technology, revised and expanded, 2nd ed.; Pizzi A., Mittal K. L., Eds.; Marcel Dekker Inc.: New York, 2003; pp 53–68.
-
- Brantseva T. V.; Antonov S. V.; Gorbunova I. Y. Adhesion Properties of the Nanocomposites Filled with Aluminosilicates and Factors Affecting Them: A Review. Int. J. Adhes. Adhes. 2018, 82, 263–281. 10.1016/j.ijadhadh.2018.01.001. - DOI
- Frigione M.; Lettieri M. Recent Advances and Trends of Nanofilled/Nanostructured Epoxies. Materials (Basel) 2020, 13 (15), 3415.10.3390/ma13153415. - DOI - PMC - PubMed
- Osman M. A.; Mittal V.; Morbidelli M.; Suter U. W. Polyurethane Adhesive Nanocomposites as Gas Permeation Barrier. Macromolecules 2003, 36 (26), 9851–9858. 10.1021/ma035077x. - DOI
- Prolongo S. G.; Gude M. R.; Ureña A. Chapter 3: Nanoreinforced Adhesives. In Nanofibers; Kumar A., Ed.; Intec, 2010; pp 39–68.
- Gokul Ganesh M.; Lavenya K.; Kirubashini K. A.; Ajeesh G.; Bhowmik S.; Epaarachchi J. A.; Yuan X. Electrically Conductive Nano Adhesive Bonding: Futuristic Approach for Satellites and Electromagnetic Interference Shielding. Adv. Aircr. Spacecr. Sci. 2017, 4 (6), 729–744. 10.12989/aas.2017.4.6.729. - DOI
-
- Fu S. Y.; Feng X. Q.; Lauke B.; Mai Y. W. Effects of Particle Size, Particle/Matrix Interface Adhesion and Particle Loading on Mechanical Properties of Particulate-Polymer Composites. Compos. Part B Eng. 2008, 39 (6), 933–961. 10.1016/j.compositesb.2008.01.002. - DOI
- Camargo P. H. C.; Satyanarayana K. G.; Wypych F. Nanocomposites: Synthesis, Structure, Properties and New Application Opportunities. Mater. Res. 2009, 12 (1), 1–39. 10.1590/S1516-14392009000100002. - DOI
-
- Tutunchi A.; Kamali R.; Kianvash A. Effect of Al2O3 Nanoparticles on the Steel-Glass/Epoxy Composite Joint Bonded by a Two-Component Structural Acrylic Adhesive. Soft Mater. 2016, 14 (1), 1–8. 10.1080/1539445X.2014.1003269. - DOI
-
- Jojibabu P.; Zhang Y. X.; Prusty B. G. A Review of Research Advances in Epoxy-Based Nanocomposites as Adhesive Materials. Int. J. Adhes. Adhes. 2020, 96, 102454.10.1016/j.ijadhadh.2019.102454. - DOI
- Bhattacharya M. Polymer Nanocomposites-A Comparison between Carbon Nanotubes, Graphene, and Clay as Nanofillers. Materials (Basel). 2016, 9 (4), 262.10.3390/ma9040262. - DOI - PMC - PubMed
- Rane A. V.; Kanny K.; Abitha V. K.; Thomas S.. Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites; Elsevier Ltd., 2018.
- Mondal T.; Ashkar R.; Butler P.; Bhowmick A. K.; Krishnamoorti R. Graphene Nanocomposites with High Molecular Weight Poly (ε -Caprolactone) Grafts : Controlled Synthesis and Accelerated Crystallization. ACS Macro Lett. 2016, 5 (3), 278–282. 10.1021/acsmacrolett.5b00930. - DOI - PubMed
- Mondal T.; Basak S.; Bhowmick A. K. Ionic Liquid Modification of Graphene Oxide and Its Role towards Controlling the Porosity, and Mechanical Robustness of Polyurethane Foam. Polymer (Guildf) 2017, 127, 106–118. 10.1016/j.polymer.2017.08.054. - DOI
Publication types
LinkOut - more resources
Full Text Sources

