Synthesis, Characterization, and Biological Activities of Novel Vanadium(IV) and Cobalt(II) Complexes
- PMID: 35155932
- PMCID: PMC8829937
- DOI: 10.1021/acsomega.1c06205
Synthesis, Characterization, and Biological Activities of Novel Vanadium(IV) and Cobalt(II) Complexes
Abstract
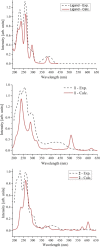
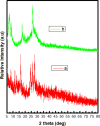
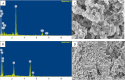
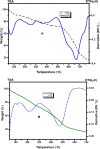
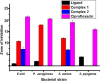
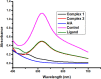
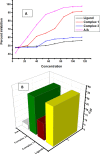
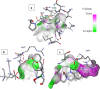
Herein, we report novel Co(II) and V(IV) complexes synthesized from an (E)-2-(((2-((2-hydroxyethyl)amino)quinolin-3-yl)methylene)amino)ethan-1-ol ligand (L), cobalt(II) chloride hexahydrate, and vanadyl(IV) sulfate in methanolic solutions. The ligand and the complexes were characterized by 1H NMR spectroscopy,13C NMR spectroscopy, UV-visible spectroscopy, fluorescence spectroscopy, FT-IR spectroscopy, powder X-ray diffraction (PXRD), scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM-EDX), mass spectroscopy (MS), thermal analysis, and molar conductance. The FT-IR spectral data showed that the ligand adopted a tridentate fashion when binding with the metal ions via the nitrogen atoms of the imine (C=N) and amine (N-H), and the oxygen atom of the hydroxyl group (O-H). The PXRD and SEM results indicated that the complexes are amorphous in nature. The density functional theory (DFT) calculated absorption and IR spectra agree very well with the corresponding experimental results. The antibacterial activities of the free ligand and its complexes were evaluated using a paper disk diffusion method. The complexes have better percent activitiy index than the free ligand. The cobalt complex exhibited a more recognizable antibacterial activity than the vanadium complex, specifically against Pseudomonas aeruginosa with a mean inhibition zone of 18.62 ± 0.19 mm, when compared with the positive control, ciprofloxacin, with a mean inhibition zone of 22.98 ± 0.08 mm at the same concentration. Furthermore, the antioxidant activities of the free ligand and its metal complexes were also determined in vitro using 2,2-diphenyl-1-picrylhydrazyl. The ligand exhibited less in vitro antioxidant activity than its transition metal complexes, in which the cobalt complex has a better antioxidant activity with half-inhibitory concentrations (IC50 of 16.01 μg/mL) than the ligand and the vanadium complex. Quantum molecular descriptors from the DFT calculations further support the experimental results. Molecular docking analysis also shed more light on the biological activities of the novel cobalt and vanadium complexes.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Hamdani H. E.; Amane M. E. Preparation, Spectral, Antimicrobial Properties and Anticancer Molecular Docking Studies of New Metal Complexes [M(Caffeine)4] (PF6)2; M = Fe(II), Co(II), Mn(II), Cd(II), Zn(II), Cu(II), Ni(II). J. Mol. Struct. 2019, 1184, 262–270. 10.1016/j.molstruc.2019.02.049. - DOI
-
- Al-Hazmi G. A.; Abou-Melha K. S.; Althagafi I.; El-Metwaly N.; Shaaban F.; Abdul Galil M. S.; El-Bindary A. A. Synthesis and Structural Characterization of Oxovanadium(IV) Complexes of Dimedone Derivatives. Appl. Organomet. Chem. 2020, 34, e567210.1002/aoc.5672. - DOI
-
- El-Gammal O. A.; Mohamed F. S.; Rezk G. N.; El-Bindary A. A. Synthesis, Characterization, Catalytic, DNA Binding and Antibacterial Activities of Co(II), Ni(II) and Cu(II) Complexes with New Schiff Base Ligand. J. Mol. Liq. 2021, 326, 115223.10.1016/j.molliq.2020.115223. - DOI
-
- El-Gammal O. A.; Mohamed F. S.; Rezk G. N.; El-Bindary A. A. Structural Characterization and Biological Activity of a New Metal Complexes Based of Schiff Base. J. Mol. Liq. 2021, 330, 4125–4136. 10.1016/j.molliq.2021.115522. - DOI
-
- Kongot M.; Reddy D. S.; Singh V.; Patel R.; Singhal N. K.; Kumar A. Oxidovanadium (IV) and Iron (III) Complexes with O2N2 Donor Linkage as Plausible Antidiabetic Candidates: Synthesis, Structural Characterizations, Glucose Uptake and Model Biological Media Studies. Appl. Organomet. Chem. 2020, 34, 1–12. 10.1002/aoc.5327. - DOI
LinkOut - more resources
Full Text Sources

