ATRI EDC: a novel cloud-native remote data capture system for large multicenter Alzheimer's disease and Alzheimer's disease-related dementias clinical trails
- PMID: 35156002
- PMCID: PMC8826990
- DOI: 10.1093/jamiaopen/ooab119
ATRI EDC: a novel cloud-native remote data capture system for large multicenter Alzheimer's disease and Alzheimer's disease-related dementias clinical trails
Erratum in
-
Corrigendum to: ATRI EDC: a novel cloud-native remote data capture system for large multicenter Alzheimer's disease and Alzheimer's disease-related dementias clinical trials.JAMIA Open. 2022 Feb 24;5(1):ooac008. doi: 10.1093/jamiaopen/ooac008. eCollection 2022 Apr. JAMIA Open. 2022. PMID: 35224459 Free PMC article.
Abstract
Objective: The Alzheimer's Therapeutic Research Institute (ATRI) developed a novel clinical data management system, the ATRI electronic data capture system (ATRI EDC), to address the complex regulatory, operational, and data requirements that arise in the conduct of multicenter Alzheimer's disease and Alzheimer's disease-related dementias (AD/ADRDs) clinical trials. We describe the system, its utility, and the broader implications for the field of clinical trials and clinical research informatics.
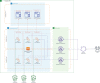
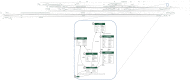
Materials and methods: The ATRI EDC system was developed, tested, and validated using community-based agile software development methods and cloud-native single-page application design principles. It offers an increasing number of application modules, supports a high degree of study-specific configuration, and empowers study teams to effectively communicate and collaborate on the accurate and timely completion of study activities.
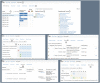
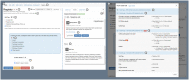
Results: To date, the ATRI EDC system supports 10 clinical studies, collecting study data for 4596 participants. Three case descriptions further illustrate how the system's capabilities support diverse study-specific requirements.
Discussion: The ATRI EDC system has several advantages: its modular capabilities can accommodate rapidly evolving research designs and technologies; its community-based agile development approach and community-friendly licensing model encourage collaboration per the principles of open science; finally, with continued development and community building efforts, the system has the potential to facilitate the effective conduct of clinical studies beyond the field of AD/ADRD.
Conclusion: By effectively addressing the requirements of multicenter AD/ADRD studies, the ATRI EDC system supports ATRI's scientific mission of rigorously testing new AD/ADRD therapies and facilitating the effective conduct of multicenter clinical studies.
Keywords: Alzheimer disease; clinical research informatics; clinical trials; data management; remote electronic data capture.
© The Author(s) 2022. Published by Oxford University Press on behalf of the American Medical Informatics Association.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
