Circadian Synchrony: Sleep, Nutrition, and Physical Activity
- PMID: 35156088
- PMCID: PMC8830366
- DOI: 10.3389/fnetp.2021.732243
Circadian Synchrony: Sleep, Nutrition, and Physical Activity
Abstract
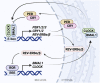
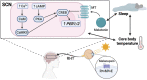
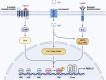
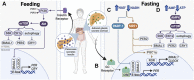
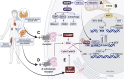
The circadian clock in mammals regulates the sleep/wake cycle and many associated behavioral and physiological processes. The cellular clock mechanism involves a transcriptional negative feedback loop that gives rise to circadian rhythms in gene expression with an approximately 24-h periodicity. To maintain system robustness, clocks throughout the body must be synchronized and their functions coordinated. In mammals, the master clock is located in the suprachiasmatic nucleus (SCN) of the hypothalamus. The SCN is entrained to the light/dark cycle through photic signal transduction and subsequent induction of core clock gene expression. The SCN in turn relays the time-of-day information to clocks in peripheral tissues. While the SCN is highly responsive to photic cues, peripheral clocks are more sensitive to non-photic resetting cues such as nutrients, body temperature, and neuroendocrine hormones. For example, feeding/fasting and physical activity can entrain peripheral clocks through signaling pathways and subsequent regulation of core clock genes and proteins. As such, timing of food intake and physical activity matters. In an ideal world, the sleep/wake and feeding/fasting cycles are synchronized to the light/dark cycle. However, asynchronous environmental cues, such as those experienced by shift workers and frequent travelers, often lead to misalignment between the master and peripheral clocks. Emerging evidence suggests that the resulting circadian disruption is associated with various diseases and chronic conditions that cause further circadian desynchrony and accelerate disease progression. In this review, we discuss how sleep, nutrition, and physical activity synchronize circadian clocks and how chronomedicine may offer novel strategies for disease intervention.
Keywords: asynchrony; circadian misalignment; circadian rhythm; entrainment; synchronization.
Conflict of interest statement
Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Abou-Samra A. B., Jüppner H., Force T., Freeman M. W., Kong X. F., Schipani E., et al. (1992). Expression Cloning of a Common Receptor for Parathyroid Hormone and Parathyroid Hormone-Related Peptide from Rat Osteoblast-like Cells: a Single Receptor Stimulates Intracellular Accumulation of Both cAMP and Inositol Trisphosphates and Increases Intracellular Free Calcium. Proc. Natl. Acad. Sci. 89, 2732–2736. 10.1073/pnas.89.7.2732 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

