A Practical Guide to the Treatment of Dravet Syndrome with Anti-Seizure Medication
- PMID: 35156171
- PMCID: PMC8927048
- DOI: 10.1007/s40263-022-00898-1
A Practical Guide to the Treatment of Dravet Syndrome with Anti-Seizure Medication
Abstract
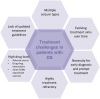
Dravet syndrome is a severe developmental and epileptic encephalopathy characterised by refractory seizures and cognitive dysfunction. The treatment is challenging, not least because the seizures are highly drug resistant, requiring multiple anti-seizure medications (ASMs), while some ASMs can exacerbate seizures. Initial treatments include the broad-spectrum ASMs valproate (VPA), and clobazam (CLB) in some regions; however, they are generally insufficient to control seizures. With this in mind, three adjunct ASMs have been approved specifically for the treatment of seizures in patients with Dravet syndrome: stiripentol (STP) in 2007 in the European Union and 2018 in the USA, cannabidiol (CBD) in 2018/2019 (in combination with CLB in the European Union) and fenfluramine (FFA) in 2020. These "add-on" therapies (mostly to VPA/CLB) are used as escalation therapies, with the choice dependent on availability in different countries, patient characteristics and caregiver preferences. Topiramate is also frequently used, with evidence of efficacy in Dravet syndrome, and there is anecdotal evidence of efficacy with bromide, which is frequently used in Germany and Japan. With a growing treatment landscape for Dravet syndrome, there can be practical challenges for clinicians, particularly with issues associated with polypharmacy. This practical guide provides an overview of these main ASMs including their indications/contraindications, mechanism of action, efficacy, safety and tolerability profile, dosage requirements, and laboratory and clinical parameters to be evaluated. Standard laboratory and clinical parameters include blood counts, liver function tests, serum concentrations of ASMs, monitoring the growth of children, as well as weight loss and acceleration of behavioural problems. Regular cardiac monitoring is also important with FFA as it has previously been associated with cases of cardiac valve disease when used in adults at high doses (up to 120 mg/day) in combination with phentermine as a therapy for obesity. Importantly, no signs of heart valve disease have been documented to date at the low doses used in patients with developmental and epileptic encephalopathies. In addition, potential drug-drug interactions and their consequences are a key consideration in everyday practice. Interactions that potentially require dosage adjustments to alleviate adverse events include the following: STP + CLB resulting in increased plasma concentrations of CLB and its active metabolite norclobazam may increase somnolence, and an interaction with STP and VPA may increase gastrointestinal adverse events. Cannabidiol has a bi-directional interaction with CLB producing an increase in plasma concentrations of 7-OH-CBD and norclobazam resulting in the potential for increased somnolence and sedation. In addition, CBD is associated with elevations of liver transaminases particularly in patients taking concomitant VPA. The interaction between FFA and STP requires a dose reduction of FFA. Furthermore, concomitant administration of VPA with topiramate has been associated with encephalopathy and/or hyperammonaemia. Finally, we briefly describe other ASMs used in Dravet syndrome, and current key clinical trials.
© 2022. The Author(s).
Conflict of interest statement
A. Strzelczyk reports personal fees and grants from Angelini Pharma/Arvelle Therapeutics, Desitin Arzneimittel, Eisai, GW Pharmaceuticals, Marinus Pharmaceuticals, UCB Pharma, UNEEG medical, and Zogenix. S. Schubert-Bast reports personal fees from Eisai, Desitin Pharma, GW Pharmaceuticals, LivaNova, UCB Pharma, and Zogenix.
Figures


References
-
- Strzelczyk A, Schubert-Bast S. Therapeutic advances in Dravet syndrome: a targeted literature review. Expert Rev Neurother. 2020;20(10):1065–1079. - PubMed
-
- Dravet C. The core Dravet syndrome phenotype. Epilepsia. 2011;52(Suppl. 2):3–9. - PubMed
-
- Selvarajah A, Zulfiqar-Ali Q, Marques P, Rong M, Andrade DM. A systematic review of adults with Dravet syndrome. Seizure. 2021;87:39–45. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

