Arrested in Glass: Actin within Sophisticated Architectures of Biosilica in Sponges
- PMID: 35156333
- PMCID: PMC9009123
- DOI: 10.1002/advs.202105059
Arrested in Glass: Actin within Sophisticated Architectures of Biosilica in Sponges
Abstract
Actin is a fundamental member of an ancient superfamily of structural intracellular proteins and plays a crucial role in cytoskeleton dynamics, ciliogenesis, phagocytosis, and force generation in both prokaryotes and eukaryotes. It is shown that actin has another function in metazoans: patterning biosilica deposition, a role that has spanned over 500 million years. Species of glass sponges (Hexactinellida) and demosponges (Demospongiae), representatives of the first metazoans, with a broad diversity of skeletal structures with hierarchical architecture unchanged since the late Precambrian, are studied. By etching their skeletons, organic templates dominated by individual F-actin filaments, including branched fibers and the longest, thickest actin fiber bundles ever reported, are isolated. It is proposed that these actin-rich filaments are not the primary site of biosilicification, but this highly sophisticated and multi-scale form of biomineralization in metazoans is ptterned.
Keywords: actin; biological materials; biomineralization; biosilica; sponges.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

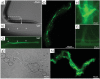
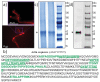


References
-
- Telford M. J., Moroz L. L., Halanych K. M., Nature 2016, 529, 286. - PubMed
-
- Simion P., Philippe H., Baurain D., Jager M., Richter D. J., Di Franco A., Roure B., Satoh N., Quéinnec É., Ereskovsky A., Lapébie P., Corre E., Delsuc F., King N., Wörheide G., Manuel M., Curr. Biol. 2017, 27, 958. - PubMed
-
- Systema Porifera: A Guide to the Classification of Sponges (Eds: Hooper J. N. A., van Soest R. W. M.), Kluwer Academic/Plenum Publishers, Dordrecht: 2002.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
