A guanosine tetraphosphate (ppGpp) mediated brake on photosynthesis is required for acclimation to nitrogen limitation in Arabidopsis
- PMID: 35156611
- PMCID: PMC8887892
- DOI: 10.7554/eLife.75041
A guanosine tetraphosphate (ppGpp) mediated brake on photosynthesis is required for acclimation to nitrogen limitation in Arabidopsis
Abstract
Guanosine pentaphosphate and tetraphosphate (together referred to as ppGpp) are hyperphosphorylated nucleotides found in bacteria and the chloroplasts of plants and algae. In plants and algae artificial ppGpp accumulation can inhibit chloroplast gene expression, and influence photosynthesis, nutrient remobilization, growth, and immunity. However, it is so far unknown whether ppGpp is required for abiotic stress acclimation in plants. Here, we demonstrate that ppGpp biosynthesis is necessary for acclimation to nitrogen starvation in Arabidopsis. We show that ppGpp is required for remodeling the photosynthetic electron transport chain to downregulate photosynthetic activity and for protection against oxidative stress. Furthermore, we demonstrate that ppGpp is required for coupling chloroplastic and nuclear gene expression during nitrogen starvation. Altogether, our work indicates that ppGpp is a pivotal regulator of chloroplast activity for stress acclimation in plants.
Keywords: A. thaliana; chloroplast; nitrogen; photoprotection; photosynthesis; plant biology; ppGpp.
© 2022, Romand et al.
Conflict of interest statement
SR, HA, CL, MB, MD, BK, SC, JC, SD, HF, SC, MH, BF No competing interests declared
Figures

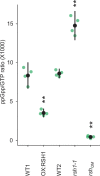

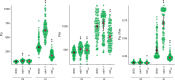
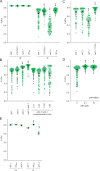


References
-
- Abdelkefi H, Sugliani M, Ke H, Harchouni S, Soubigou-Taconnat L, Citerne S, Mouille G, Fakhfakh H, Robaglia C, Field B. Guanosine tetraphosphate modulates salicylic acid signalling and the resistance of Arabidopsis thaliana to Turnip mosaic virus. Molecular Plant Pathology. 2018;19:634–646. doi: 10.1111/mpp.12548. - DOI - PMC - PubMed
-
- Andrews S. FastQC. A quality control tool for high throughput sequence data. Version 0.11.5FastQC. 2010 https://www.bioinformatics.babraham.ac.uk/projects/fastqc

