Updates in ANCA-associated vasculitis
- PMID: 35156630
- PMCID: PMC12462881
- DOI: 10.5152/eujrheum.2022.20248
Updates in ANCA-associated vasculitis
Abstract
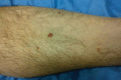
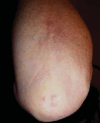
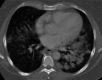
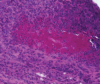
Antineutrophil cytoplasm antibody (ANCA)-associated vasculitides (AAV) are small-vessel vasculitides that include granulomatosis with polyangiitis (formerly Wegener's granulomatosis), microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis (Churg - Strauss syndrome). Renal-limited AAV can be considered a fourth entity. Despite their rarity and still unknown cause(s), research into AAV has been very active over the past decades and has allowed for the development of new therapeutic regimens. The pathogenesis is a complex process of immune dysregulations with genetic and environmental influences. Recent genome-wide association studies have identified multiple genetic predisposing variants, especially at the major histocompatibility complex region. The pathogenic role of antimyeloperoxidase ANCA (MPO-ANCA) is well supported by several animal models, but that of antiproteinase 3 ANCA (PR3-ANCA) is not as strongly demonstrated. B cells likely play a major role in the pathogenesis because they produce ANCAs, as do neutrophil abnormalities, imbalances in T-cell subtypes, and/or cytokine - chemokine networks. The role of the alternative complement pathway was established more recently, and studies of the antagonist of human C5a receptor (avacopan) in AAV have just been completed, with promising results. The current standard management of severe AAV still consists of remission induction therapy with glucocorticoids combined with rituximab or, less often now, cyclophosphamide. Several studies showed that reduced-dose regimens of glucocorticoids are noninferior to the previously used heavier regimens, for therefore less cumulative exposure to glucocorticoids. Avacopan use may even lead to new steroid-free therapeutic approaches, at least for some selected patients. Several trials and studies have now shown the superiority of rituximab over azathioprine or methotrexate as maintenance therapy. However, the optimal dosing regimen and duration for maintenance remain to be better defined, at the individual patient level. Many changes have occurred in the standard of care for AAV over the past decades, and more are expected soon, including with use of avacopan, but also, likely, a few other agents under investigation or development.
Figures




References
-
- Jennette JC, Falk RJ, Bacon PA, et al. Revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65(1):1–11. - PubMed
-
- Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 2010;33(8):1094–1100. - PubMed
-
- Leavitt RY, Fauci AS, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis Rheum. 2010;33(8):1101–1107. - PubMed
-
- Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37(2):187–192. - PubMed
-
- Craven A, Robson J, Ponte C, et al. ACR/EULARendorsed study to develop diagnostic and classification criteria for vasculitis (DCVAS). Clin Exp Nephrol. 2013;17(5):619–621. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

