Dlx1/2-dependent expression of Meis2 promotes neuronal fate determination in the mammalian striatum
- PMID: 35156680
- PMCID: PMC8918808
- DOI: 10.1242/dev.200035
Dlx1/2-dependent expression of Meis2 promotes neuronal fate determination in the mammalian striatum
Abstract
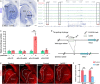
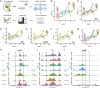
The striatum is a central regulator of behavior and motor function through the actions of D1 and D2 medium-sized spiny neurons (MSNs), which arise from a common lateral ganglionic eminence (LGE) progenitor. The molecular mechanisms of cell fate specification of these two neuronal subtypes are incompletely understood. Here, we found that deletion of murine Meis2, which is highly expressed in the LGE and derivatives, led to a large reduction in striatal MSNs due to a block in their differentiation. Meis2 directly binds to the Zfp503 and Six3 promoters and is required for their expression and specification of D1 and D2 MSNs, respectively. Finally, Meis2 expression is regulated by Dlx1/2 at least partially through the enhancer hs599 in the LGE subventricular zone. Overall, our findings define a pathway in the LGE whereby Dlx1/2 drives expression of Meis2, which subsequently promotes the fate determination of striatal D1 and D2 MSNs via Zfp503 and Six3.
Keywords: Dlx1/2; Meis2; Six3; Zfp503; LGE; Mouse; Striatum.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures
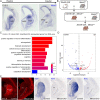
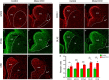
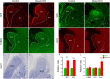
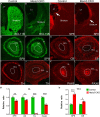


Similar articles
-
SP8 and SP9 coordinately promote D2-type medium spiny neuron production by activating Six3 expression.Development. 2018 Jul 23;145(14):dev165456. doi: 10.1242/dev.165456. Development. 2018. PMID: 29967281 Free PMC article.
-
Homeobox Gene Six3 is Required for the Differentiation of D2-Type Medium Spiny Neurons.Neurosci Bull. 2021 Jul;37(7):985-998. doi: 10.1007/s12264-021-00698-5. Epub 2021 May 20. Neurosci Bull. 2021. PMID: 34014554 Free PMC article.
-
Meis2 is a Pax6 co-factor in neurogenesis and dopaminergic periglomerular fate specification in the adult olfactory bulb.Development. 2014 Jan;141(1):28-38. doi: 10.1242/dev.097295. Epub 2013 Nov 27. Development. 2014. PMID: 24284204
-
Dynamic changes in the transcriptional profile of subventricular zone-derived postnatally born neuroblasts.Mech Dev. 2013 Jun-Aug;130(6-8):424-32. doi: 10.1016/j.mod.2012.11.003. Epub 2012 Dec 5. Mech Dev. 2013. PMID: 23220001 Review.
-
Transcriptional regulation of olfactory bulb neurogenesis.Anat Rec (Hoboken). 2013 Sep;296(9):1364-82. doi: 10.1002/ar.22733. Epub 2013 Jul 31. Anat Rec (Hoboken). 2013. PMID: 23904336 Review.
Cited by
-
Epigenomic profiling of mouse nucleus accumbens at single-cell resolution.Mol Cell Neurosci. 2023 Sep;126:103857. doi: 10.1016/j.mcn.2023.103857. Epub 2023 May 1. Mol Cell Neurosci. 2023. PMID: 37137383 Free PMC article.
-
Transcription factors in microcephaly.Front Neurosci. 2023 Nov 29;17:1302033. doi: 10.3389/fnins.2023.1302033. eCollection 2023. Front Neurosci. 2023. PMID: 38094004 Free PMC article. Review.
-
Transcription factor Sp9 is a negative regulator of D1-type MSN development.Cell Death Discov. 2022 Jun 30;8(1):301. doi: 10.1038/s41420-022-01088-0. Cell Death Discov. 2022. PMID: 35773249 Free PMC article.
-
Human striatal glia differentially contribute to AD- and PD-specific neurodegeneration.Nat Aging. 2023 Mar;3(3):346-365. doi: 10.1038/s43587-023-00363-8. Epub 2023 Feb 9. Nat Aging. 2023. PMID: 36993867 Free PMC article.
-
Cleft palate, congenital heart disease, and developmental delay involving MEIS2 heterozygous mutations found in the patient with attention deficit hyperactivity disorder: a case report.Front Pediatr. 2024 Dec 24;12:1500152. doi: 10.3389/fped.2024.1500152. eCollection 2024. Front Pediatr. 2024. PMID: 39776641 Free PMC article.
References
-
- Anderson, S. A., Qiu, M., Bulfone, A., Eisenstat, D. D., Meneses, J., Pedersen, R. and Rubenstein, J. L. R. (1997). Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron 19, 27-37. 10.1016/S0896-6273(00)80345-1 - DOI - PubMed
-
- Bocchi, V. D., Conforti, P., Vezzoli, E., Besusso, D., Cappadona, C., Lischetti, T., Galimberti, M., Ranzani, V., Bonnal, R. J. P., De Simone, M.et al. (2021). The coding and long noncoding single-cell atlas of the developing human fetal striatum. Science 372, eabf5759. 10.1126/science.abf5759 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

