Mitochondrial energy metabolism regulates the nutrient import activity and endocytosis of APC transporters
- PMID: 35156710
- PMCID: PMC9117475
- DOI: 10.1002/1873-3468.14314
Mitochondrial energy metabolism regulates the nutrient import activity and endocytosis of APC transporters
Abstract
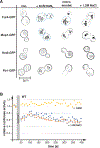
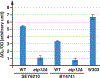
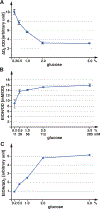
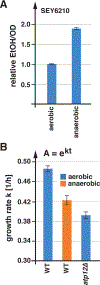
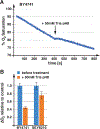
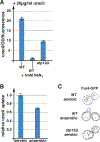
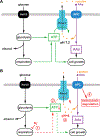
Nutrient import by APC-type transporters is predicted to have a high energy demand because it depends on the plasma membrane proton gradient established by the ATP-driven proton pump Pma1. We show that Pma1 is indeed a major energy consumer and its activity is tightly linked to the cellular ATP levels. The low Pma1 activity caused by acute loss of respiration resulted in a dramatic drop in cytoplasmic pH, which triggered the downregulation of the major proton importers, the APC transporters. This regulatory system is likely the reason for the observed rapid endocytosis of APC transporters during many environmental stresses. Furthermore, we show the importance of respiration in providing ATP to maintain a strong proton gradient for efficient nutrient uptake.
Keywords: membrane trafficking; nutrient import; respiration.
© 2022 Federation of European Biochemical Societies.
Figures

Similar articles
-
Eisosomes are metabolically regulated storage compartments for APC-type nutrient transporters.Mol Biol Cell. 2018 Aug 15;29(17):2113-2127. doi: 10.1091/mbc.E17-11-0691. Epub 2018 Jun 21. Mol Biol Cell. 2018. PMID: 29927345 Free PMC article.
-
Regulation of nutrient transporters by metabolic and environmental stresses.Curr Opin Cell Biol. 2020 Aug;65:35-41. doi: 10.1016/j.ceb.2020.02.009. Epub 2020 Mar 19. Curr Opin Cell Biol. 2020. PMID: 32200208 Free PMC article. Review.
-
Compensatory Internalization of Pma1 in V-ATPase Mutants in Saccharomyces cerevisiae Requires Calcium- and Glucose-Sensitive Phosphatases.Genetics. 2018 Feb;208(2):655-672. doi: 10.1534/genetics.117.300594. Epub 2017 Dec 18. Genetics. 2018. PMID: 29254995 Free PMC article.
-
Endocytosis of nutrient transporters in fungi: The ART of connecting signaling and trafficking.Comput Struct Biotechnol J. 2021 Mar 19;19:1713-1737. doi: 10.1016/j.csbj.2021.03.013. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 33897977 Free PMC article. Review.
-
Proton Transport and pH Control in Fungi.Adv Exp Med Biol. 2016;892:33-68. doi: 10.1007/978-3-319-25304-6_3. Adv Exp Med Biol. 2016. PMID: 26721270 Free PMC article. Review.
Cited by
-
The Impact of the Competition on miRNA, Proteins, and Metabolites in the Blood Exosomes of the Yili Horse.Genes (Basel). 2025 Feb 15;16(2):224. doi: 10.3390/genes16020224. Genes (Basel). 2025. PMID: 40004554 Free PMC article.
-
Mevalonate secretion is not mediated by a singular non-essential transporter in Saccharomyces cerevisiae.Biotechnol Notes. 2024 Oct 18;5:140-150. doi: 10.1016/j.biotno.2024.10.001. eCollection 2024. Biotechnol Notes. 2024. PMID: 39498316 Free PMC article.
-
ER-plasma membrane contact sites deliver ER lipids and proteins for rapid cell surface expansion.J Cell Biol. 2024 Dec 2;223(12):e202308137. doi: 10.1083/jcb.202308137. Epub 2024 Sep 20. J Cell Biol. 2024. PMID: 39302311 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

