Multiomic characterization of pancreatic cancer-associated macrophage polarization reveals deregulated metabolic programs driven by the GM-CSF-PI3K pathway
- PMID: 35156921
- PMCID: PMC8843093
- DOI: 10.7554/eLife.73796
Multiomic characterization of pancreatic cancer-associated macrophage polarization reveals deregulated metabolic programs driven by the GM-CSF-PI3K pathway
Abstract
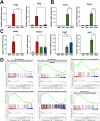
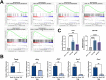
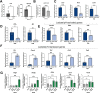
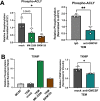
The pancreatic ductal adenocarcinoma microenvironment is composed of a variety of cell types and marked by extensive fibrosis and inflammation. Tumor-associated macrophages (TAMs) are abundant, and they are important mediators of disease progression and invasion. TAMs are polarized in situ to a tumor promoting and immunosuppressive phenotype via cytokine signaling and metabolic crosstalk from malignant epithelial cells and other components of the tumor microenvironment. However, the specific distinguishing features and functions of TAMs remain poorly defined. Here, we generated tumor-educated macrophages (TEMs) in vitro and performed detailed, multiomic characterization (i.e., transcriptomics, proteomics, metabolomics). Our results reveal unique genetic and metabolic signatures of TEMs, the veracity of which were queried against our in-house single-cell RNA sequencing dataset of human pancreatic tumors. This analysis identified expression of novel, metabolic TEM markers in human pancreatic TAMs, including ARG1, ACLY, and TXNIP. We then utilized our TEM model system to study the role of mutant Kras signaling in cancer cells on TEM polarization. This revealed an important role for granulocyte-macrophage colony-stimulating factor (GM-CSF) and lactate on TEM polarization, molecules released from cancer cells in a mutant Kras-dependent manner. Lastly, we demonstrate that GM-CSF dysregulates TEM gene expression and metabolism through PI3K-AKT pathway signaling. Collectively, our results define new markers and programs to classify pancreatic TAMs, how these are engaged by cancer cells, and the precise signaling pathways mediating polarization.
Keywords: cancer biology; human; immunology; inflammation; metabolomics; mouse; pancreatic cancer; proteomics; tumor-associated macrophages.
© 2022, Boyer et al.
Conflict of interest statement
SB, HL, NS, LZ, PS, AA, MW, RS, VB, YZ, AN, MP, CH No competing interests declared, CL CAL has received consulting fees from Astellas Pharmaceuticals and Odyssey Therapeutics and is an inventor on patents pertaining to Kras regulated metabolic pathways, redox control pathways in pancreatic cancer, and targeting the GOT1-pathway as a therapeutic approach (US Patent No: 2015126580-A1, 05/07/2015; US Patent No: 20190136238, 05/09/2019; International Patent No: WO2013177426-A2, 04/23/2015)
Figures









References
-
- Baardman J, Verberk SGS, van der Velden S, Gijbels MJJ, van Roomen C, Sluimer JC, Broos JY, Griffith GR, Prange KHM, van Weeghel M, Lakbir S, Molenaar D, Meinster E, Neele AE, Kooij G, de Vries HE, Lutgens E, Wellen KE, de Winther MPJ, Van den Bossche J. Macrophage ATP citrate lyase deficiency stabilizes atherosclerotic plaques. Nature Communications. 2020;11:6296. doi: 10.1038/s41467-020-20141-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- R00 CA241357/CA/NCI NIH HHS/United States
- P30 CA046592/CA/NCI NIH HHS/United States
- U24 CA210967/CA/NCI NIH HHS/United States
- F32 CA228328/CA/NCI NIH HHS/United States
- R01 CA248160/CA/NCI NIH HHS/United States
- T32 CA140044/CA/NCI NIH HHS/United States
- R01 GM094231/GM/NIGMS NIH HHS/United States
- R01 CA244931/CA/NCI NIH HHS/United States
- P30 DK034933/DK/NIDDK NIH HHS/United States
- P30 CA062203/CA/NCI NIH HHS/United States
- U24 DK097153/DK/NIDDK NIH HHS/United States
- U01 CA224145/CA/NCI NIH HHS/United States
- K99 CA241357/CA/NCI NIH HHS/United States
- R50 CA232985/CA/NCI NIH HHS/United States
- R37 CA237421/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

