Serial ultrathin sections to identify ultrastructural localization of GLUT1 molecules in vesicles in brain endothelial cells-correlative light and electron microscopy in depth
- PMID: 35157050
- PMCID: PMC8973401
- DOI: 10.1093/jmicro/dfac005
Serial ultrathin sections to identify ultrastructural localization of GLUT1 molecules in vesicles in brain endothelial cells-correlative light and electron microscopy in depth
Abstract
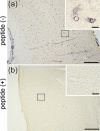
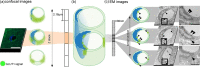
Precise immunolocalization of molecules in relation to ultrastructural features is challenging, especially when the target is small and not frequent enough to be included in tiny ultrathin sections randomly selected for electron microscopy (EM). Glucose transporter 1 (GLUT1) is in charge of transporting glucose across brain capillary endothelial cells (BCECs). Paraformaldehyde-fixed floating sections (50 μm thick) of mouse brain were immunolabeled with anti-GLUT1 antibody and visualized with fluoronanogold. Fluorescent images encompassing the entire hemisphere were tiled to enable selection of GLUT1-positive BCECs suitable for subsequent EM and landmark placement with laser microdissection to guide trimming. Sections were then fixed with glutaraldehyde, gold enhanced to intensify the labeling and fixed with osmium tetroxide to facilitate ultrastructural recognition. Even though a region that contained target BCECs was successfully trimmed in the resin block, it was only after observation of serial ultrathin sections that GLUT1 signals in coated vesicles on the same cross section corresponding to the cross section preidentified by confocal laser microscope. This is the first ultrastructural demonstration of GLUT1 molecules in coated vesicles, which may well explain its functional relevance to transport glucose across BCECs. Successful ultrastructural localization of molecules in relation to well-preserved target structure in native tissue samples, as achieved in this study, will pave the way to understand the functional relevance of molecules and their relation to ultrastructural details.
Keywords: CLEM; GLUT1; blood–brain barrier; coated vesicle; pre-embedding method; serial section.
© The Author(s) 2022. Published by Oxford University Press on behalf of The Japanese Society of Microscopy.
Figures




References
-
- Anraku Y, Kuwahara H, Fukusato Y, Mizoguchi A, Ishii T, Nitta K, Matsumoto Y, Toh K, Miyata K, Uchida S, Nishina K, Osada K, Itaka K, Nishiyama N, Mizusawa H, Yamasoba T, Yokota T, and Kataoka K (2017) Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain. Nat. Commun. 8: 1001. - PMC - PubMed
-
- Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, and Terasaki T (2011) Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J. Neurochem. 117: 333–345. - PubMed
-
- Farrell C L and Pardridge W M (1991) Ultrastructural localization of blood-brain barrier-specific antibodies using immunogold-silver enhancement techniques. J. Neurosci. Methods 37: 103–110. - PubMed
-
- Cornford E M, Hyman S, and Pardridge W M (1993) An electron microscopic immunogold analysis of developmental up-regulation of the blood-brain barrier GLUT1 glucose transporter. J. Cereb. Blood Flow Metab. 13: 841–854. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

