Molecular and Serologic Investigation of the 2021 COVID-19 Case Surge Among Vaccine Recipients in Mongolia
- PMID: 35157057
- PMCID: PMC8845004
- DOI: 10.1001/jamanetworkopen.2021.48415
Molecular and Serologic Investigation of the 2021 COVID-19 Case Surge Among Vaccine Recipients in Mongolia
Abstract
This cross-sectional study uses molecular and serologic methods to investigate the 2021 surge in COVID-19 cases among vaccine recipients in Mongolia.
Conflict of interest statement
Figures
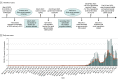
References
-
- Ritchie H, Mathieu E, Rodés-Guirao L, et al. . Coronavirus pandemic (COVID-19). Accessed October 15, 2021. https://ourworldindata.org/coronavirus
-
- Eckhardt CM, Cummings MJ, Rajagopalan KN, et al. . Evaluating the efficacy and safety of human anti-SARS-CoV-2 convalescent plasma in severely ill adults with COVID-19: a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21(1):499. doi:10.1186/s13063-020-04422-y - DOI - PMC - PubMed
-
- Tada T, Zhou H, Samanovic MI, et al. . Comparison of neutralizing antibody titers elicited by mRNA and adenoviral vector vaccine against SARS-CoV-2 variants. bioRxiv 2021. . doi:10.1101/2021.07.19.452771 - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

