Non-autonomous stomatal control by pavement cell turgor via the K+ channel subunit AtKC1
- PMID: 35157082
- PMCID: PMC9048897
- DOI: 10.1093/plcell/koac038
Non-autonomous stomatal control by pavement cell turgor via the K+ channel subunit AtKC1
Abstract
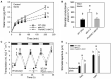
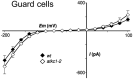
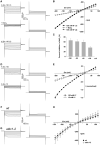
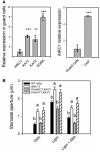
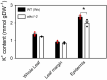
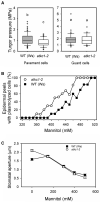
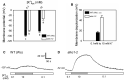
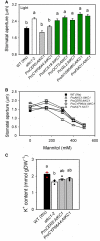
Stomata optimize land plants' photosynthetic requirements and limit water vapor loss. So far, all of the molecular and electrical components identified as regulating stomatal aperture are produced, and operate, directly within the guard cells. However, a completely autonomous function of guard cells is inconsistent with anatomical and biophysical observations hinting at mechanical contributions of epidermal origins. Here, potassium (K+) assays, membrane potential measurements, microindentation, and plasmolysis experiments provide evidence that disruption of the Arabidopsis thaliana K+ channel subunit gene AtKC1 reduces pavement cell turgor, due to decreased K+ accumulation, without affecting guard cell turgor. This results in an impaired back pressure of pavement cells onto guard cells, leading to larger stomatal apertures. Poorly rectifying membrane conductances to K+ were consistently observed in pavement cells. This plasmalemma property is likely to play an essential role in K+ shuttling within the epidermis. Functional complementation reveals that restoration of the wild-type stomatal functioning requires the expression of the transgenic AtKC1 at least in the pavement cells and trichomes. Altogether, the data suggest that AtKC1 activity contributes to the building of the back pressure that pavement cells exert onto guard cells by tuning K+ distribution throughout the leaf epidermis.
© American Society of Plant Biologists 2022. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Comment in
-
Mind the context: K+ channel subunit AtKC1 tunes local osmotic environment to adjust stomatal movement.Plant Cell. 2022 Apr 26;34(5):1884-1885. doi: 10.1093/plcell/koac048. Plant Cell. 2022. PMID: 35182153 Free PMC article. No abstract available.
Similar articles
-
Stomatal action directly feeds back on leaf turgor: new insights into the regulation of the plant water status from non-invasive pressure probe measurements.Plant J. 2010 Jun 1;62(6):1072-82. doi: 10.1111/j.1365-313X.2010.04213.x. Epub 2010 Mar 25. Plant J. 2010. PMID: 20345603
-
Stomatal Spacing Safeguards Stomatal Dynamics by Facilitating Guard Cell Ion Transport Independent of the Epidermal Solute Reservoir.Plant Physiol. 2016 Sep;172(1):254-63. doi: 10.1104/pp.16.00850. Epub 2016 Jul 11. Plant Physiol. 2016. PMID: 27406168 Free PMC article.
-
KIN7 Kinase Regulates the Vacuolar TPK1 K+ Channel during Stomatal Closure.Curr Biol. 2018 Feb 5;28(3):466-472.e4. doi: 10.1016/j.cub.2017.12.046. Epub 2018 Jan 27. Curr Biol. 2018. PMID: 29395926
-
Stomatal development in the context of epidermal tissues.Ann Bot. 2021 Jul 30;128(2):137-148. doi: 10.1093/aob/mcab052. Ann Bot. 2021. PMID: 33877316 Free PMC article. Review.
-
Signaling Transduction of ABA, ROS, and Ca2+ in Plant Stomatal Closure in Response to Drought.Int J Mol Sci. 2022 Nov 26;23(23):14824. doi: 10.3390/ijms232314824. Int J Mol Sci. 2022. PMID: 36499153 Free PMC article. Review.
Cited by
-
Automated 3D segmentation of guard cells enables volumetric analysis of stomatal biomechanics.Patterns (N Y). 2022 Nov 9;3(12):100627. doi: 10.1016/j.patter.2022.100627. eCollection 2022 Dec 9. Patterns (N Y). 2022. PMID: 36569557 Free PMC article.
-
Mechanisms of salinity tolerance and their possible application in the breeding of vegetables.BMC Plant Biol. 2023 Mar 14;23(1):139. doi: 10.1186/s12870-023-04152-8. BMC Plant Biol. 2023. PMID: 36915096 Free PMC article. Review.
-
A Promoter Collection for Cell-Targeted Analysis Within the Stomatal Complex.Plant Direct. 2025 Feb 12;9(2):e70045. doi: 10.1002/pld3.70045. eCollection 2025 Feb. Plant Direct. 2025. PMID: 39943924 Free PMC article.
-
Identification of Shaker Potassium Channel Family Members and Functional Characterization of SsKAT1.1 in Stenotaphrum secundatum Suggest That SsKAT1.1 Contributes to Cold Resistance.Int J Mol Sci. 2024 Aug 31;25(17):9480. doi: 10.3390/ijms25179480. Int J Mol Sci. 2024. PMID: 39273427 Free PMC article.
-
Mind the context: K+ channel subunit AtKC1 tunes local osmotic environment to adjust stomatal movement.Plant Cell. 2022 Apr 26;34(5):1884-1885. doi: 10.1093/plcell/koac048. Plant Cell. 2022. PMID: 35182153 Free PMC article. No abstract available.
References
-
- Bauer H, Ache P, Lautner S, Fromm J, Hartung W, Al-Rasheid KA, Sonnewald S, Sonnewald U, Kneitz S, Lachmann N, et al. (2013) The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr Biol 23: 53–57 - PubMed
-
- Blatt MR (2000) Cellular signaling and volume control in stomatal movements in plants. Annu Rev Cell Dev Biol 16: 221–241 - PubMed
-
- Britto DT, Coskun D, Kronzucker HJ (2021) Potassium physiology from Archean to Holocene: A higher-plant perspective. J Plant Physiol 262: 153432. - PubMed
-
- Clough SJ, Bent AF (1998) Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

