The Incidence of SARS-CoV-2 Reinfection in Persons With Naturally Acquired Immunity With and Without Subsequent Receipt of a Single Dose of BNT162b2 Vaccine : A Retrospective Cohort Study
- PMID: 35157493
- PMCID: PMC8855786
- DOI: 10.7326/M21-4130
The Incidence of SARS-CoV-2 Reinfection in Persons With Naturally Acquired Immunity With and Without Subsequent Receipt of a Single Dose of BNT162b2 Vaccine : A Retrospective Cohort Study
Abstract
Background: There is insufficient evidence regarding the magnitude and durability of protection conferred by a combined effect of naturally acquired immunity after SARS-CoV-2 infection and vaccine-induced immunity.
Objective: To compare the incidence rate of SARS-CoV-2 reinfection in previously infected persons to that of previously infected persons who subsequently received a single dose of BNT162b2 messenger RNA vaccine.
Design: A retrospective cohort study emulating a randomized controlled target trial through a series of nested trials.
Setting: Nationally centralized database of Maccabi Healthcare Services, Israel.
Participants: Persons with documented SARS-CoV-2 infection who did not receive subsequent SARS-CoV-2 vaccination were compared with persons with documented SARS-CoV-2 infection who received a single dose of the BNT162b2 vaccine at least 3 months after infection.
Intervention: Forty-one randomized controlled trials were emulated, in which 107 413 Maccabi Healthcare Services' members aged 16 years and older were eligible for at least 1 trial.
Measurements: SARS-CoV-2-related outcomes of infection, symptomatic disease, hospitalization, and death, between 2 March and 13 December 2021.
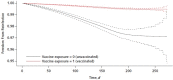
Results: A statistically significant decreased risk (hazard ratio, 0.18 [95% CI, 0.15 to 0.20]) for reinfection was found among persons who were previously infected and then vaccinated versus those who were previously infected but remained unvaccinated. In addition, there was a decreased risk for symptomatic disease (hazard ratio, 0.24 [CI, 0.20 to 0.29]) among previously infected and vaccinated persons compared with those who were not vaccinated after infection. No COVID-19-related mortality cases were found.
Limitation: Hybrid protection against non-Delta variants could not be inferred.
Conclusion: Persons previously infected with SARS-CoV-2 gained additional protection against reinfection and COVID-19 from a subsequent single dose of the BNT162b2 vaccine. Nonetheless, even without a subsequent vaccination, reinfection appeared relatively rare.
Primary funding source: None.
Conflict of interest statement
Figures


References
-
- Haas EJ , Angulo FJ , McLaughlin JM , et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819-1829. [PMID: ] doi:10.1016/S0140-6736(21)00947-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
