SWOG 1318: A Phase II Trial of Blinatumomab Followed by POMP Maintenance in Older Patients With Newly Diagnosed Philadelphia Chromosome-Negative B-Cell Acute Lymphoblastic Leukemia
- PMID: 35157496
- PMCID: PMC9084435
- DOI: 10.1200/JCO.21.01766
SWOG 1318: A Phase II Trial of Blinatumomab Followed by POMP Maintenance in Older Patients With Newly Diagnosed Philadelphia Chromosome-Negative B-Cell Acute Lymphoblastic Leukemia
Abstract
Purpose: Chemotherapy outcomes in older patients with Philadelphia (Ph) chromosome-negative B-acute lymphoblastic leukemia (ALL) are very poor. Here, we evaluated blinatumomab as induction and consolidation therapy followed by prednisone, vincristine, 6-mercaptopurine, and methotrexate (POMP) maintenance chemotherapy in this patient population.
Patients and methods: Patients were treated at National Clinical Trial Network sites. Eligibility criteria included age ≥ 65 years and newly diagnosed Ph chromosome-negative B-ALL. Patients received blinatumomab as induction for one-two cycles until attainment of response (complete remission (CR) and CR with incomplete count recovery). Patients then received three cycles of consolidation with blinatumomab followed by 18 months of POMP maintenance chemotherapy. Eight doses of intrathecal methotrexate were administered as central nervous system prophylaxis.
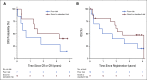
Results: Twenty-nine eligible patients were enrolled. The median age was 75 years, and the median bone marrow blast count at diagnosis was 87%. Cytogenetic risk was poor in 10 patients (34%), and five of 14 patients (36%) tested had the Ph-like ALL gene signature. Nineteen patients (66%; 95% CI, 46 to 82) achieved CR. Kaplan-Meier 3-year disease-free survival and overall survival estimates were 37% (95% CI, 17 to 57) and 37% (95% CI, 20 to 55), respectively.
Conclusion: Blinatumomab was well tolerated and effective in the treatment of older patients with newly diagnosed Ph chromosome-negative B-ALL, including patients with poor-risk cytogenetics. The 3-year disease-free survival and overall survival results are encouraging and suggest that this approach should be further explored.
Conflict of interest statement
Figures




References
-
- National Cancer Institute : SEER stat fact sheet: Acute lymphocytic leukemia. Bethesda, MD, 2012. http://seer.cancer.gov/statfacts/html/alyl.html
-
- Stock W: personal communication. Data from 5 CALGB trials 1988-2001
-
- Larson RA: The US trials in adult acute lymphoblastic leukemia. Ann Hematol 83:S127-S128, 2004. (suppl I) - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UG1 CA233290/CA/NCI NIH HHS/United States
- UG1 CA233230/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- UG1 CA232760/CA/NCI NIH HHS/United States
- UG1 CA233180/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- UG1 CA233247/CA/NCI NIH HHS/United States
- UG1 CA233253/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U10 CA180819/CA/NCI NIH HHS/United States
- P30 CA014236/CA/NCI NIH HHS/United States
- UG1 CA233327/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

