Genotypic and Phenotypic Spectrum of Foveal Hypoplasia: A Multicenter Study
- PMID: 35157951
- PMCID: PMC9341240
- DOI: 10.1016/j.ophtha.2022.02.010
Genotypic and Phenotypic Spectrum of Foveal Hypoplasia: A Multicenter Study
Abstract
Purpose: To characterize the genotypic and phenotypic spectrum of foveal hypoplasia (FH).
Design: Multicenter, observational study.
Participants: A total of 907 patients with a confirmed molecular diagnosis of albinism, PAX6, SLC38A8, FRMD7, AHR, or achromatopsia from 12 centers in 9 countries (n = 523) or extracted from publicly available datasets from previously reported literature (n = 384).
Methods: Individuals with a confirmed molecular diagnosis and availability of foveal OCT scans were identified from 12 centers or from the literature between January 2011 and March 2021. A genetic diagnosis was confirmed by sequence analysis. Grading of FH was derived from OCT scans.
Main outcome measures: Grade of FH, presence or absence of photoreceptor specialization (PRS+ vs. PRS-), molecular diagnosis, and visual acuity (VA).
Results: The most common genetic etiology for typical FH in our cohort was albinism (67.5%), followed by PAX6 (21.8%), SLC38A8 (6.8%), and FRMD7 (3.5%) variants. AHR variants were rare (0.4%). Atypical FH was seen in 67.4% of achromatopsia cases. Atypical FH in achromatopsia had significantly worse VA than typical FH (P < 0.0001). There was a significant difference in the spectrum of FH grades based on the molecular diagnosis (chi-square = 60.4, P < 0.0001). All SLC38A8 cases were PRS- (P = 0.003), whereas all FRMD7 cases were PRS+ (P < 0.0001). Analysis of albinism subtypes revealed a significant difference in the grade of FH (chi-square = 31.4, P < 0.0001) and VA (P = 0.0003) between oculocutaneous albinism (OCA) compared with ocular albinism (OA) and Hermansky-Pudlak syndrome (HPS). Ocular albinism and HPS demonstrated higher grades of FH and worse VA than OCA. There was a significant difference (P < 0.0001) in VA between FRMD7 variants compared with other diagnoses associated with FH.
Conclusions: We characterized the phenotypic and genotypic spectrum of FH. Atypical FH is associated with a worse prognosis than all other forms of FH. In typical FH, our data suggest that arrested retinal development occurs earlier in SLC38A8, OA, HPS, and AHR variants and later in FRMD7 variants. The defined time period of foveal developmental arrest for OCA and PAX6 variants seems to demonstrate more variability. Our findings provide mechanistic insight into disorders associated with FH and have significant prognostic and diagnostic value.
Keywords: AHR; Albinism; Aniridia; FHONDA; FRMD7; Foveal hypoplasia; GPR143; Genetics; Genotype-phenotype correlation; Hermansky–Pudlak syndrome; OCT; PAX6; Retinal development; SLC38A8.
Copyright © 2022 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


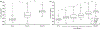
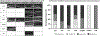
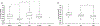
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

