Cerebrospinal fluid p-tau231 as an early indicator of emerging pathology in Alzheimer's disease
- PMID: 35158308
- PMCID: PMC8850760
- DOI: 10.1016/j.ebiom.2022.103836
Cerebrospinal fluid p-tau231 as an early indicator of emerging pathology in Alzheimer's disease
Abstract
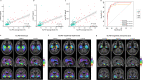
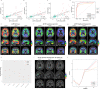
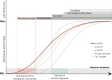
Phosphorylated tau (p-tau) epitopes in cerebrospinal fluid (CSF) are accurate biomarkers for a pathological and clinical diagnosis of Alzheimer's disease (AD) and are seen to be increased in preclinical stage of the disease. However, it is unknown if these increases transpire earlier, prior to amyloid-beta (Aβ) positivity as determined by position emission tomography (PET), and if an ordinal sequence of p-tau epitopes occurs at this incipient phase METHODS: We measured CSF concentrations of p-tau181, p-tau217 and p-tau231 in 171 participants across the AD continuum who had undergone Aβ ([18F]AZD4694) and tau ([18F]MK6240) position emission tomography (PET) and clinical assessment FINDINGS: All CSF p-tau biomarkers were accurate predictors of cognitive impairment but CSF p-tau217 demonstrated the largest fold-changes in AD patients in comparison to non-AD dementias and cognitively unimpaired individuals. CSF p-tau231 and p-tau217 predicted Aβ and tau to a similar degree but p-tau231 attained abnormal levels first. P-tau231 was sensitive to the earliest changes of Aβ in the medial orbitofrontal, precuneus and posterior cingulate before global Aβ PET positivity was reached INTERPRETATION: We demonstrate that CSF p-tau231 increases early in development of AD pathology and is a principal candidate for detecting incipient Aβ pathology for therapeutic trial application FUNDING: Canadian Institutes of Health Research (CIHR), Canadian Consortium of Neurodegeneration and Aging, Weston Brain Institute, Brain Canada Foundation, the Fonds de Recherche du Québec.
Keywords: Alzheimer's disease; Amyloid; Cerebrospinal fluid; Phosphorylated tau; Positron emission tomography; Preclinical.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
Declaration of interests EVM is the Chief scientific officer in ADx Neurosciences. ES and CF are employees of ADx NeuroSciences. SG has served as a consultant for TauRx, Biogen Canada Member and Roche Canada. HZ has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics and CogRx, and has given lectures in symposia sponsored by Fujirebio, Alzecure and Biogen. Alzheimer's Association Global Biomarker Standardization Consortium chair and cofounder of Brain Biomarker Solutions in gothenburg AB (BBS) which is a part of the GU ventures Incubator program. KB has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. CF, and ES and are employee of ADx NeuroSciences, Gent, Belgium, EVM is a co-founder of ADx NeuroSciences. The other authors declare no competing interest.
Figures

References
-
- Scheltens P., Blennow K., Breteler M.M., et al. Alzheimer's disease. Lancet. 2016;388(10043):505–517. - PubMed
-
- Olsson B., Lautner R., Andreasson U., et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15(7):673–684. - PubMed
-
- Hansson O., Zetterberg H., Buchhave P., Londos E., Blennow K., Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5(3):228–234. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

